Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Prabhanjan S Giram and Version 2 by Rita Xu.
Eudragit, synthesized by radical polymerization, is used for enteric coating, precise temporal release, and targeting the entire gastrointestinal system. Evonik Healthcare Germany offers different grades of Eudragit. The ratio of methacrylic acid to its methacrylate-based monomers used in the polymerization reaction defines the final product’s characteristics and consequently its potential range of applications.
- Eudragit classification
- Eudragit synthesis
- drug delivery
1. Introduction
In the last decades, natural or synthetic polymers have made a significant difference in painting, cosmetics, bio-imaging, trade, and medicine. The contributions of polymer science and engineering to healthcare applications are showing promise. These polymers have many advantages in drug delivery applications, such as being biodegradable, biocompatible, simple to eliminate from the body, minimizing the number of doses needed, maintaining the drug concentration level in the optimal range, and making patients more likely to take their medicine because the polymer coat hides the organoleptic characteristics of the formulation. According to literature studies, each polymer has some unique properties that permit the fabrication of nanotechnology-based formulation with a controlled size distribution, solubility, flexibility, and permeation for the intended application.
These properties are crucial in the development of novel polymeric carriers for the preparation of nanocarrier coating test masking site-specific release time-dependent release accomplished using multiple types of polymers to alter the release pattern or modify the release kinetics in therapeutic drug delivery application. To deliver the drug at target sites, the drug was enclosed in a polymeric shell matrix. However, some polymers have a few limitations, such as being non-compatible with active ingredients, non-biodegradable, and toxic; to overcome these problems, there is a growing concern for the synthesis of polymer by modern polymerization method with catalysis. In recent years, Eudragit has proven to be one of the most attractive areas of research due to the importance of its advanced drug delivery system. Rohm & Hass GmbH, Darmstadt, first introduced Eudragit in 1953 as an acid-resistant, alkaline-soluble drug coating functional material. Eudragit is a brand name marketed primarily by Evonik Technologies Germany.
It is an extensive collection of anionic, cationic, or neutral copolymers derived from methacrylic acid and methacrylic or acrylic esters, as well as their derivatives. According to their physicochemical properties, such copolymers synthesized using diverse polymerization techniques such as reversible addition–fragmentation chain transfer (RAFT), atom transfer radical polymerization (ATRP), and group transfer exhibit varying degrees of pH-dependent or independent solubility in numerous studies. Different varieties of Eudragit polymer are utilized to selectively target the entire GIT, including the small intestine, colon, and large intestine. These copolymers are also investigated for protein and drug protection from the stomach’s acidic environment, etc. Eudragit are nonionic and synthetic polyionic copolymers, including different concentrations of methacrylic acid esters, alkyl methacrylates, 2-(dimethylamino)ethyl methacrylate. The various concentration of non-ionized and ionized groups in the structure of these copolymers generally controls. They are typically used as film-coating agents in oral tablets as well capsule formulations for protection and to give prolonged release dosage form. In both organic and aqueous wet granulation techniques, Eudragit polymers are used as binders [1].
The next paragraph discusses the nomenclature of several grades of Eudragit polymer. Eudragit E100, E12,5, and EPO are cationic copolymer made from methyl methacrylate, dimethylaminoethyl methacrylate, and butyl methacrylate in all three grades. Poly(butyl methacrylate-co-(2-dimethylamino) ethyl methacrylate-co-methyl methacrylate) is its chemical name. The alkali range is 180 mg KOH/g, the molecular weight is roughly 47,000 g/mol, and the solubility at gastric pH 5 is low viscosity. Both Eudragit S 12,5 and S 100 are anionic copolymers that are produced from methyl methacrylate and methacrylic acid. Glass transition temperature, acid value, and molecular weight is >150 °C, 190 mg KOH/g, 125,000 g/mol, and soluble in basic pH at 7.0. Soluble Eudragit FS 30D is an anionic copolymer of methacrylic acid, methyl acrylate, and methyl methacrylate. Glass transition temperature, acid value, and molecular weight is >150 °C, 190 mg KOH/g, 125,000 g/mol, and soluble in basic pH at 7.0. Soluble Eudragit FS 30D is an anionic copolymer of methacrylic acid, methyl acrylate, and methyl methacrylate. It is soluble in above pH 7 but insoluble in acid pH. Glass temperature, molecular weight, and acid value are 48 °C, 280,000 g/mol, and 70 mg KOH/g. Eudragit L12,5 and L 100 anionic derived methyl methacrylic acid and methacrylic acid have the same molecular weight, 125,000 g/mol, glass transition temperature of more than 150 °C acid value of 315 mg KOH/g, and soluble above pH 6. Eudragit RL100, RL 30 D, and RLPO are copolymers containing methyl methacrylate and ethyl acrylate with quaternary ammonium groups and less methacrylate acid. RL 30D grade has low viscosity and a milky-white appearance. Its glass transition temperatures are 40 °C and 55 °C, and its molecular weight is 32,000 g/mol. Eudragit RL 100 and RLPO share a 70 °C glass temperature, 32,000 g/mol molecular weight, and 28.1 mg KOH/g acid value [2].
2. Classification of Eudragit Polymer
Many different grades of Eudragit polymer usually available are granules, dry powder, organic solvent, and aqueous dispersion forms. A mixture of acetone and isopropanol in a ratio of 40:60 is highly used as the organic solvent. The chemical compositions, characteristic feature of different types of Eudragit, are shown in Table 1.Table 1. Classification of polymethacrylate based on polymeric grades.
| Eudragit Grade | Applications | Chemical Composition | Solubility |
|---|---|---|---|
| Cationic (Aminoalkylmethacrylate copolymers) Eudragit E 12.5 Eudragit E 100 |
Increased geriatric and pediatric patient compliance. Increased bioavailability and dissolution profile. Increased anti-inflammatory action. High oral bioavailability |
Poly(butyl methacrylate, (2-dimethyl aminoethyl) methacrylate, methyl methacrylate) 1:2:1 Poly(butyl methacrylate, (2-dimethyl aminoethyl) methacrylate, methyl methacrylate) 1:2:1 |
Both are soluble in gastric fluid to pH 5 |
| Anionic (Methacrylic acid copolymers) Eudragit L 100 Eudragit L 100-55 Eudragit L 12.5 Eudragit L 12.5 P Eudragit S 12.5 Eudragit S 12.5 P Eudragit L 30 D-55 Eudragit S 100 Eudragit FS 30 D |
pH-dependent and high release. Increased oral absorption. Increased taste masking. Controlled release. Colonic-specific drug delivery. Targeting drug delivery. Delay release profile. High oral bioavailability |
Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, ethyl acrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:2 Poly(methacrylic acid, methyl methacrylate) 1:2 Poly(methacrylic acid, ethyl acrylate) 1:1 Poly(methacrylic acid, methyl methacrylate) 1:2 Methyl acrylate, methyl methacrylate, and methacrylic acid |
Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 5.5 Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 6 Soluble in intestinal fluid around pH 7 Soluble in intestinal fluid around pH 7 Soluble in intestinal fluid from pH 5.5 Soluble in intestinal fluid around pH 7 Soluble above pH 6.8 |
| Neutral (Methacrylic acid copolymers) 1. Eudragit RL PO 2. Eudragit RL 30 D 3. Eudragit RL 100 (Type A) 4. Eudragit RS PO 5. Eudragit RS 30 D 6. Eudragit RS 100 (Type B) |
Increased release time and ocular bioavailability. Increased shelf life for ophthalmic dosage form. Sustainable drug release for more than 6 h. Sustained release with significance for vaginal drug delivery. Improved permeation and increased bioavailability as well as shelf life. |
Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.2 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 Poly(ethyl acrylate, methyl methacrylate, 2-trimethylammonioethyl methacrylate chloride” or “2-(methacryloyloxy)-N,N,N-trimethylethanaminium chloride) 1:2:0.1 |
Permeability is High Permeability is High Permeability is High Permeability is Low Permeability is Low Permeability is Low |
| Neutral (Methacrylic acid copolymers) 1. Eudragit NM 30 D 2. Eudragit NE 30 D 3. Eudragit NE 40 D |
Poly(ethyl acrylate, methyl methacrylate) with 0.7% (PEG stearyl ether) 2:1 Poly(ethyl acrylate, methyl methacrylate) with 1.5% (nonoxynol) 2:1 Poly(ethyl acrylate, methyl methacrylate) with 1.5% (nonoxynol) 2:1 |
Permeable, swellable Permeable, swellable Permeable, swellable |
3. Characterization of Eudragit
Glass transition temperature, X-ray particle diffraction, DSC, FT-IR, physiological buffer differential, and pH-sensitive properties distinguish Eudragit grades. As indicated in Table 2, a differential thermal study of these polymers indicates a single thermal start as the glass transition temperature, which is characteristic of the various Eudragit grades. The glass transition temperature affects pharmaceutical dosage from storage conditions, film production, melt processing, etc. Due to the amorphous Eudragit structure, it exhibits prolonged release. Small molecules of drugs, solvents, or plasticizers lower the glass transition temperature; these properties are important for pharmaceutical applications. Triethyl citrate is used as the main plasticizer in Eudragit [4][5][4,5]. X-ray powder diffractograms of Eudragit S 100, L 100, RS, and RL are shown in Figure 1. Based on the literature study, Eudragit grades indicate the amorphous nature of the polymers [6][7][6,7]. TGA curves with DSC thermograms of the Eudragit L30D, L as well as S are explained in Figure 2. Here, reswearchers can see thermal characteristics of the Eudragit L30D, L, and S from DSC curves appeared to be compatible with the reflectance data by DSC/FT-IR microspectroscopy. Figure 3 are presented three-dimension FT-IR spectra of Eudragit L30D, L, and S. The spectral ranges of these three polymers appear at 3100 and 2850 cm−1, 1800 and 1650 cm−1, and 1350 and 900 cm−1. The C-H starching bend peak range is between 3100 and 2850 cm−1, the C=O stretching vibration groups is between 1800 and 1650 cm−1, and C-O stretching vibration mode is between 1350 and 900 cm−1 [8][9][8,9]. The diffraction of all these Eudragit grades shows a halo(gaussian), suggesting that the polymers are amorphous, as seen in Figure 1.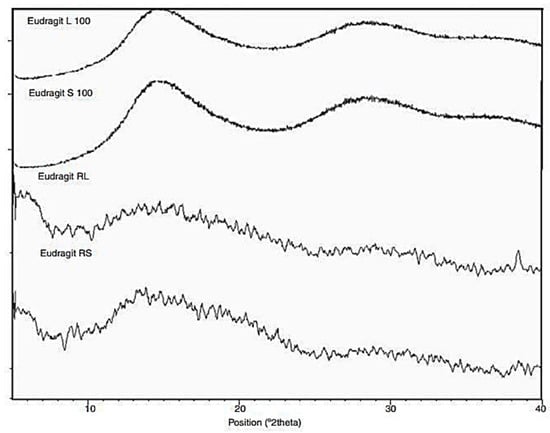
Figure 1. X-ray diffractogram of various Eudragit polymers, Eudragit L 100, S 100, Eudragit RL, and Eudragit RS.
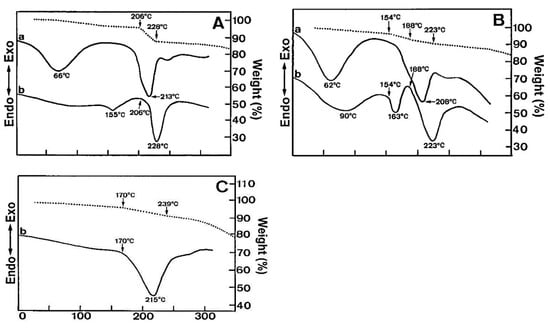
Figure 2.
Temperature dependencies of the FT-IR peak intensity for the various IR bands of Eudragits L, S, and L30D. (
A
) Eudragit L; (
B
) Eudragit S; (
C
) Eudragit L30D.
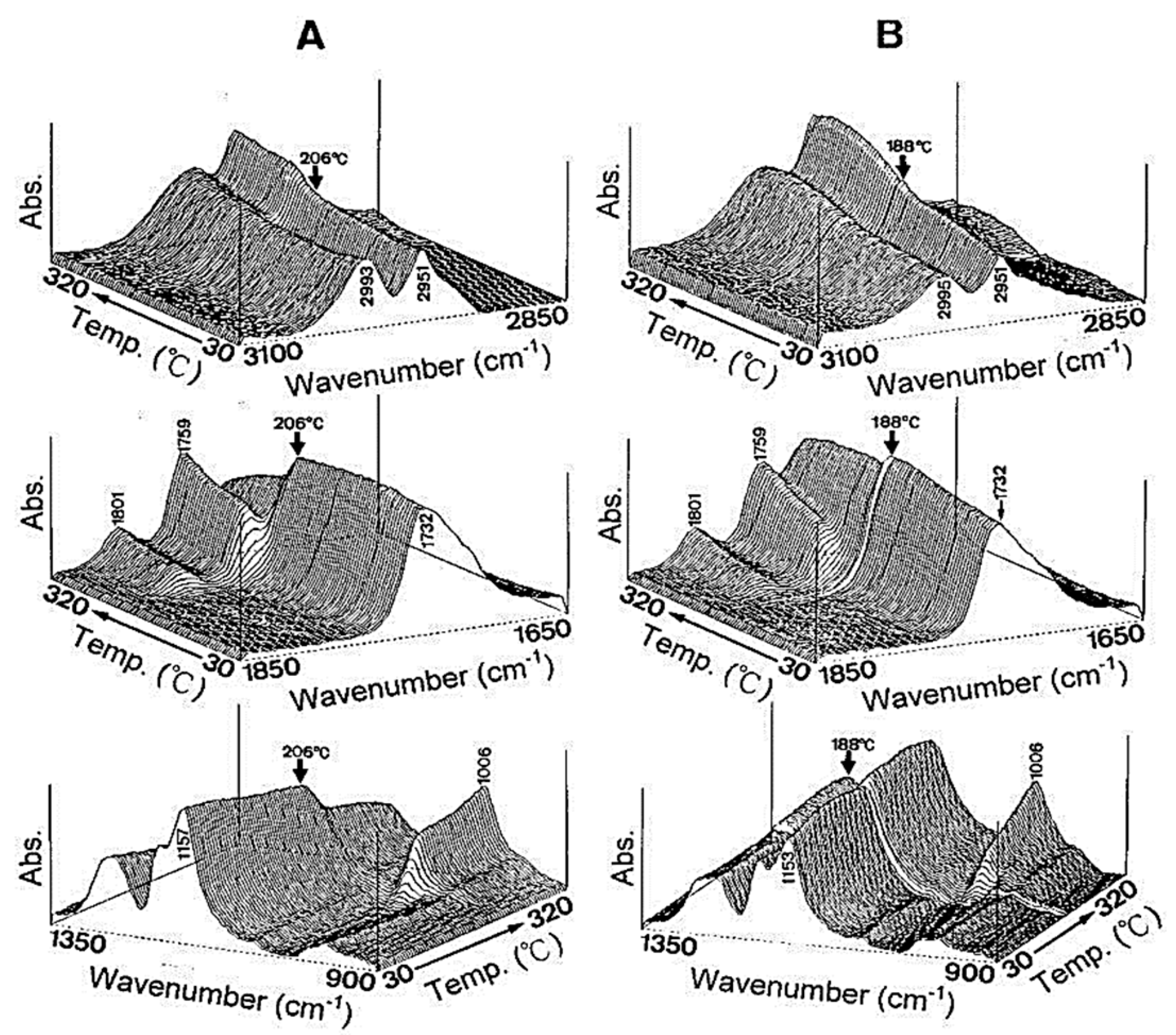
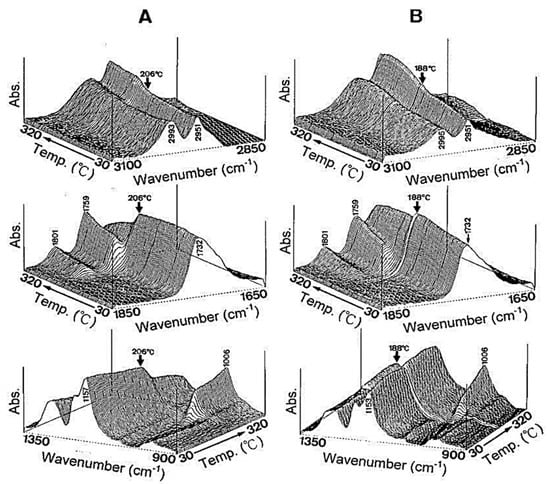
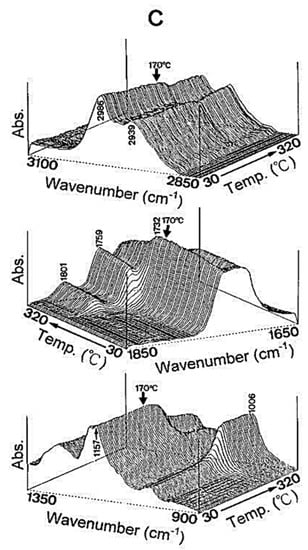
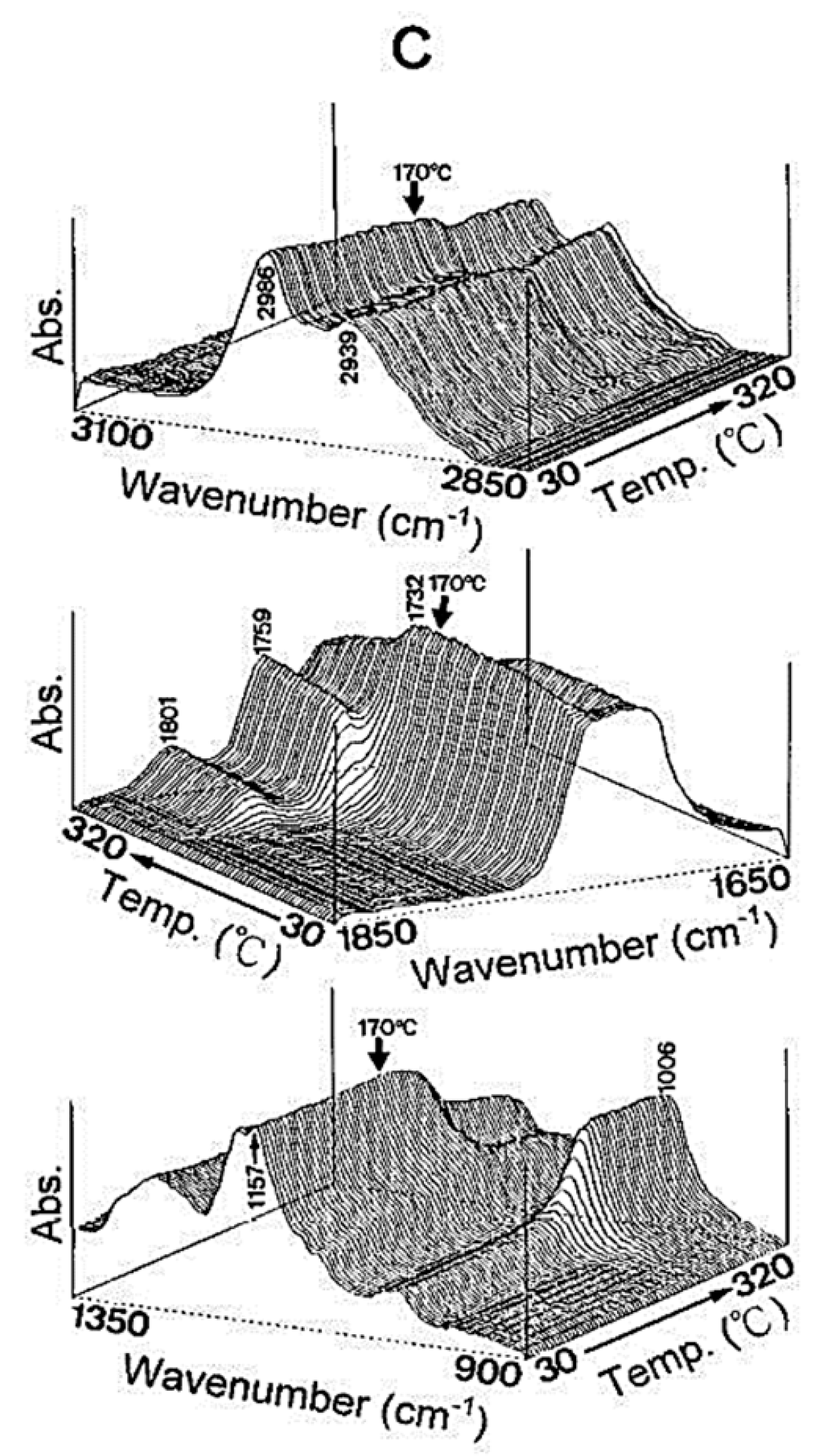
Figure 3.
Three-dimensional plots of reflectance FT-IR spectra of Eudragits L, S, and respect to temperature. (
A
) Eudragit L; (
B
) Eudragit S; (
C
) Eudragit L30D.
Table 2. Glass transition temperature of Eudragit with various grades.
| Grades of Eudragit Polymer | Glass Transition Temperature (°C) |
|---|---|
| Eudragit E 100/E PO | 48 |
| Eudragit FS 30 D | 48 |
| Eudragit NE 30 D | 9 |
| Eudragit ME 30 D | 11 |
| Eudragit L 100-55 | 110 |
| Eudragit RL 100 | 70 |
| Eudragit RS 100 | 65 |
4. Synthesis of Eudragit Polymer
Although gastrointestinal drug release is the ideal drug delivery route, there are several problems when the medicine is injected into the digestive system. Limitation such as reduction in drug bioavailability is observed during drug transport through the gastrointestinal mucosa [10][11][10,11]. In general, drug delivery on the oral mucosa is difficult because of a constant flow of saliva and mobility of the tissue, limiting the residence time of drugs administrated to the oral cavity. The size of a buccal dosage form is restricted by the very limited area available for the application of the delivery system. This size restriction, in turn, limits the amount of drugs that can be incorporated into the dosage forms [12]. In order to improve intestinal drug transport, Haupstein et al. synthesized preactivated thiolated Eudragit L100-55 (Scheme 1) [13].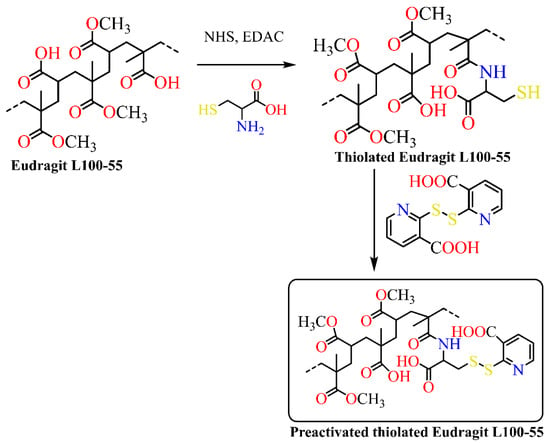
Scheme 1. Synthesis of preactivated thiolated Eudragit L100-55.
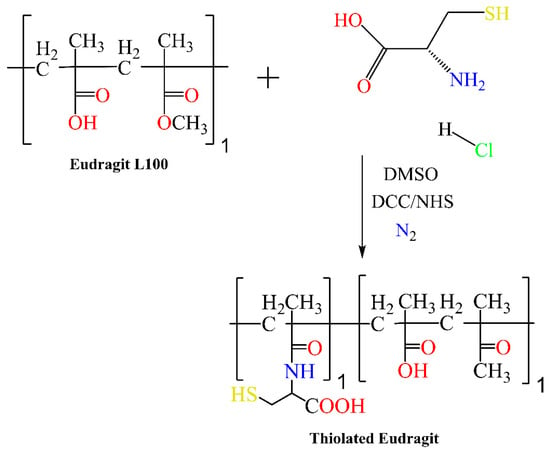
Scheme 2. One-step synthetic scheme for the preparation of thiolated Eudragit.
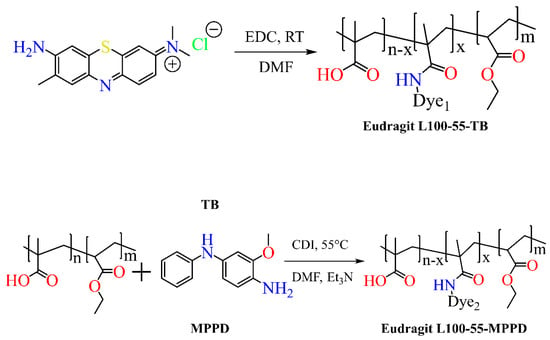
Scheme 3. Synthesis of dye-anchored Eudragit polymers.
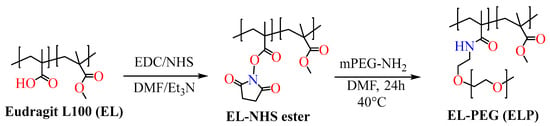
Scheme 4. Scheme for the synthesis of PEGylated Eudragit L100 polymer.
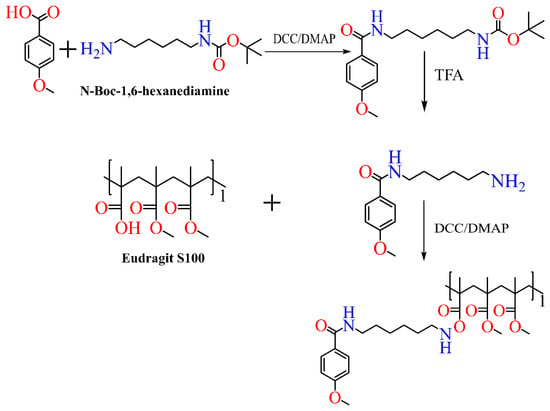
Scheme 5. Synthesis of anisamide-based Eudragit polymer.
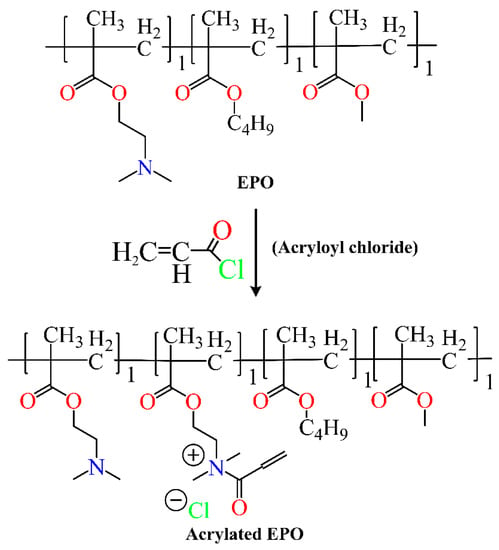
Scheme 6. Synthesis of Acrylated Eudragit polymer.
4.1. Atom Transfer Radical Polymerization
Atom transfer radical polymerization (ATRP) is normally known as a transition-metal-assisted atom transfer reaction. ATRP deals with a monomer, an initiator (composed of a movable halogen group), and a catalyst (consists of a transition metal-based ligand); hence, it is considered a multicomponent system [21]. Organic derivatives such as acrylates, acrylamides, styrenes, acrylonitrile, etc., are suitable radical stabilizers during polymer synthesis and are used as monomers in ATRP reactions. In the ATRP process, the active moieties (radicals) are produced through a transition-metal-catalyzed reversible redox reaction (Scheme 7). The process involves one electron oxidation as well as the detachment of halogen species from the inactive reactant. The combination of intermediate radicals with monomers results in the propagation of the polymer chain through kp (rate constant for propagation).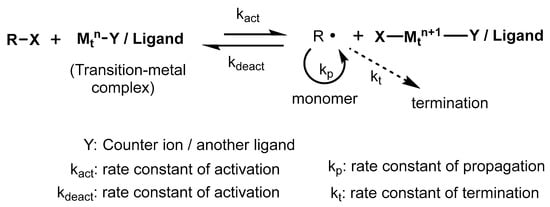
Scheme 7. General mechanism for transition-metal-catalyzed ATRP.
4.2. Reversible Addition–Fragmentation
Reversible addition–fragmentation chain transfer (RAFT) polymerization is the most diverse tool to develop various functional block copolymers as it can tolerate functional monomer diversity as well as a wide variety of reaction media [22]. The synthetic process involved in RAFT polymerization is comparatively small and easy to use. Thiocarbonylthio group is a well-known chain transfer (RAFT) agent, which accelerates degenerative chain transfer in the process. Most of the chains in the RAFT polymerization contain a terminal thiocarbonylthio group, as in the case of polymer b. In other words, a monomer unit is installed between the SR bond of the chain transfer agent to produce the polymer. The mechanism of the RAPT polymerization process is shown in Scheme 8.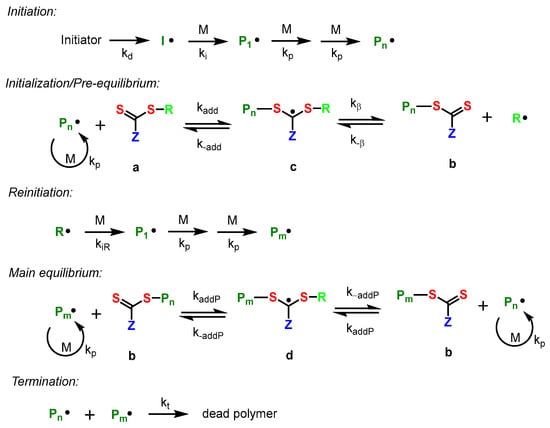
Scheme 8. Mechanism of basic equilibria process involved during RAFT polymerization.
4.3. Chain Transfer Polymerization
Interestingly, various polymer chains per catalyst propagate during chain transfer polymerization from catalyst1 to the chain transfer agent (Scheme 9) [23]. The chain transfer occurs fast in terms of propagation and is also reversible in nature. Additionally, no other termination pathways, such as βH abstraction, take place during the polymerization process. Chain transfer metal present in the macromolecular chain facilitates chemical functionalization.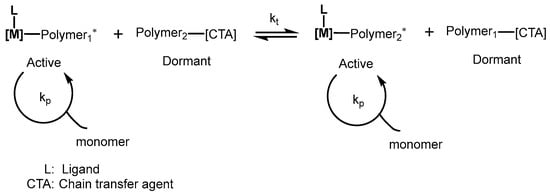
Scheme 9. Process involving chain transfer polymerization. * Scheme represented the Coordinative Chain Transfer Polymerization a process involving a dynamical equilibrium between propagating and dormant species.
