The precise control and monitoring of pH values remain critical for many chemical, physiological and biological processes. Perylene diimide (PDI)-based molecules and materials exhibit excellent thermal, chemical and photochemical stability, unique UV-vis absorption and fluorescent emission properties, low cytotoxicity, as well as intrinsic electron-withdrawing (n-type semiconductor) nature and impressive molecular assembly capability. These features combined enable promising applications of PDIs in chemosensors via optical signal modulations (e.g., fluorescent or colorimetric). One of the typical applications lies in the probing of pH under various conditions, which in turn helps monitor the extracellular (environmental) and intracellular pH change and pH-relying molecular recognition of inorganic or organic ions, as well as biological species, and so on.
- perylene diimide
- chemosensor
- pH probing
- colorimetric sensor
- fluorescent sensor
1. Introduction
2. Colorimetric Chemosensors
The colorimetric pH response of PDI-based chemosensors benefits from their strong absorptivity in the mid-visible spectral region, with molar absorption coefficient as high as 30,000–90,000 cm−1 M−1 [2,10][2][10] in the broad spectra (color) variation range [5]. However, due to the rigid perylene skeleton and the unique π-conjugation with the imide nitrogen as the node, structural substitution at the imide position generally has little influence on the spectral absorption property of PDI molecules [1]. At present, only few studies have been reported, such as those on hydrochromism [11] and pH-mediated color change [12], in this aspect, mainly due to the considerable challenge of the intense red background and poor water solubility of hydrophobic PDI core. Satisfyingly, modification at its bay area could result in considerable influence on its absorption spectra and improvement in solubility [1].2.1. Hydrochromism for pH and Humidity Sensing
Upon functionalization of PDI scaffold at its 1,7-bay position by electron-donating 3-hydroxycyclobutenedione moieties, the derivative (namely PDI−1) thus obtained showed pronounced acidity and solubility in polar solvents [11]. The intramolecular electron transfer (IET) between cyclobutene moiety and PDI backbone can be modulated significantly by the protonation/deprotonation of the -OH groups, thus resulting in bathochromic shift of the absorption peak from 570 nm (acidic form itself) to 612~667 nm (conjugate base form PDI−12− in different organic solvents). The solvent effect of the colorimetric response can also be utilized for probing the solvent polarity and humidity, which can be applied in monitoring trace water in organic solvents, such as tetrahydrofuran (THF), as well as film sensing of humidity in the gas phase. The sensor film, fabricated by immobilization of PDI−1 in polyethylene glycol (PEG) matrix, showed drastic color change from red-purple to blue-green accompanied by distinct absorption spectra change upon exposure to water moisture. Such halochromic response is instantaneous, and reversible, for which the sensor material can be regenerated after drying.2.2. Synergistic CO2 and pH Sensing
3. Fluorescent Chemosensors
3.1. pH Sensing Based on Photoinduced Electron Transfer (PET) Mechanism
3.2. pH Sensing Based on Supramolecular (De)Aggregation Mechanism
3.3. pH Sensing Based on Fluorescence Resonance Energy Transfer (FRET) Mechanism
In addition to the above-mentioned shape-defined supramolecular assemblies (e.g., nanofibers), some other supramolecular architectures can also be utilized in pH sensing. Inspired by the natural amphiphiles forming biological bilayer membranes in living vesicle systems, some amphiphilic building blocks of PDIs have been synthesized and used for chemosensors [18,33][18][31]. By functionalizing the hydrophobic skeleton of PDI with asymmetric hydrophilic and hydrophobic groups at its imide positions, amphiphilic derivatives PDI−17 and PDI−18 (Figure 1) and the sensing performance were reported [18]. Such water-soluble molecules can be co-assembled into a bilayer nanoscopic vesicle (acting as an energy acceptor) in aqueous solution, for which water-soluble energy donors can be enclosed inside the vesicle. By adjusting the spectral overlap of donors and acceptors, a controlled pH-sensitive FRET process from the “core” (encapsulated donor) to the “shell” (bilayer dye membrane) can be realized, so as to drive the optical sensing application of such nanocapsule vesicle in aqueous solutions. Ultrasensitive pH response was obtained for the nanocapsule sensor as tested in a wide range of pH 3.0–11.0; the sensing response was revealed as dramatic fluorescence color change, covering the whole visible light range under UV (366 nm) illumination.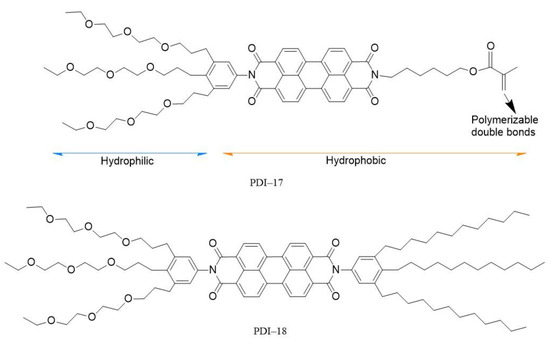
3.4. pH Sensing Based on Tunable Lateral Dimensions of 1D Nanostructures
3.5. pH Sensing Based on Volume Phase Transition of Unimolecular Micelle
Compared to the dimensional change of 1D molecular assembly, structure-controllable unimolecular micelles can also be developed into optical chemosensors reliant on the pH induced structure change. Upon substitution with sufficiently large dendrimers, PDI−20 and PDI−21, can behave as a unimolecular micelle with the core being hydrophobic and the shell hydrophobic. The globular “core–shell” morphology (average diameters around 20 nm) makes the micelles a unique platform to be used in pH probing in complete aqueous media (pH = 7) [20]. These micelles displayed reversible volume phase transition around their pKa values (6.34 and 8.05, respectively) due to the ionization (fully charged state; size increasing) or deionization (totally uncharged state; size decreasing) of the polymer chains induced by pH variation. Accompanying size change, the significant change of fluorescence can be used as sensor signals for probing the pH. It is the flexible cationic or anionic polyelectrolyte side chains as the outer shell, which contributes to high water solubility (>10 g/L) and high fluorescence quantum yields in water (0.11 for PDI−20 and 0.13 for PDI−21) by preventing the central PDI chromophore from aggregation, for which the electrostatic repulsion capability (in relation to the polymer chain stretching or collapsing) is highly dependent on pH.3.6. pH Imaging in Cells
External pH stimuli or an acid-base microenvironment are extremely crucial for cell survival as well as its internal and external physiological/pathological activities in vivo, and therefore, accurate in situ probing of the pH would help in health monitoring and disease diagnosis [9,24,34][9][24][32]. PDIs as fluorophores for pH monitoring in cells have gained particular interest mainly because of the advantages in the following aspects [13,21,22,23,24][13][21][22][23][24]: versatile molecular design, robust structure with combined chemical/photochemical/thermal stability, lower biological toxicity, high fluorescent emission brightness (intensity) under lower excitation energy, lower auto-emission and light scattering interference from intracellular microenvironments, desired cell penetration capability, etc. Further support comes from the essential light penetration across tissues and multidimensional analysis ability of the fluorescence technique itself. The key challenge remaining for PDI-based sensors lies in their hydrophobic skeletons and aggregation-induced weak emission intensity in aqueous solution. To date, amphiphilic PDIs with good solubility and biodistribution in water are the most preferable architectures for intracellular pH probing via fluorescence intensity or lifetime modulation as the signal.References
- Chen, S.; Slattum, P.; Wang, C.Y.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998.
- Wang, Q.; Li, Z.; Tao, D.D.; Zhang, Q.; Zhang, P.; Guo, D.P.; Jiang, Y.B. Supramolecular aggregates as sensory ensembles. Chem. Commun. 2016, 52, 12929–12939.
- Chen, S.; Xue, Z.X.; Gao, N.; Yang, X.M.; Zang, L. Perylene diimide-based fluorescent and colorimetric sensors for environmental detection. Sensors 2020, 20, 917.
- Ali, S.; Gupta, A.; Shafiei, M.; Langford, S.J. Recent advances in perylene diimide-based active materials in electrical mode gas sensing. Chemosensors 2021, 9, 30.
- Singh, P.; Sharma, P.; Kaur, N.; Mittal, L.S.; Kumar, K. Perylene diimides: Will they flourish as reaction-based probes? Anal. Methods 2020, 12, 3560–3574.
- Zhang, M.; Shi, J.F.; Liao, C.L.; Tian, Q.Y.; Wang, C.Y.; Chen, S.; Zang, L. Perylene imide-based optical chemosensors for vapor detection. Chemosensors 2021, 9, 1.
- Zhou, W.W.; Liu, G.; Yang, B.; Ji, Q.Y.; Xiang, W.M.; He, H.; Xu, Z.; Qi, C.D.; Li, S.; Yang, S.G.; et al. Review on application of perylene diimide (PDI)-based materials in environment: Pollutant detection and degradation. Sci. Total Environ. 2021, 780, 146483.
- Singh, P.; Hirsch, A.; Kumar, S. Perylene diimide-based chemosensors emerging in recent years: From design to sensing. TrAC Trends Anal. Chem. 2021, 138, 116237.
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chem. Rev. 2020, 120, 12357–12489.
- Aigner, D.; Borisov, S.M.; Klimant, I. New fluorescent perylene bisimide indicators—A platform for broadband pH optodes. Anal. Bioanal. Chem. 2011, 400, 2475–2485.
- Maeda, T.; Würthner, F. Halochromic and hydrochromic squaric acid functionalized perylene bisimide. Chem. Comm. 2015, 51, 7661–7664.
- Pfeifer, D.; Klimant, I.; Borisov, S.M. Ultrabright red-emitting photostable perylene bisimide dyes: New indicators for ratiometric sensing of high pH or carbon dioxide. Chem. Eur. J. 2018, 24, 10711–10720.
- Yang, L.; Liu, Y.; Li, P.; Liu, Y.L.; Liang, X.M.; Fu, Y.; Ye, F. A dual-mode colorimetric/fluorescent probe based on perylene: Response to acidic pH values. J. Taiwan Inst. Chem. Eng. 2021, 129, 97–103.
- Zang, L.; Liu, R.C.; Holman, M.W.; Nguyen, K.T.; Adams, D.M. A single-molecule probe based on intramolecular electron transfer. J. Am. Chem. Soc. 2002, 124, 10640–10641.
- Ye, F.; Liang, X.M.; Wu, N.; Li, P.; Chai, Q.; Fu, Y. A new perylene-based fluorescent pH chemosensor for strongly acidic condition. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 359–364.
- Zhang, W.; Gan, S.Y.; Li, F.H.; Han, D.X.; Zhang, Q.X.; Niu, L. pH responding reversible supramolecular self-assembly of water-soluble amino-imidazole-armed perylene diimide dye for biological applications. RSC Adv. 2015, 5, 2207–2212.
- Li, S.Y.; Long, T.; Wang, Y.; Yang, X.G. Self-assembly, protonation-dependent morphology, and photophysical properties of perylene bisimide with tertiary amine groups. Dyes Pigm. 2020, 173, 107896.
- Zhang, X.; Rehm, S.; Safont-Sempere, M.M.; Würthner, F. Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems. Nature Chem. 2009, 1, 623–629.
- Pandeeswar, M.; Govindaraju, T. Engineering molecular self-assembly of perylene diimide through pH-responsive chiroptical switching. Mol. Syst. Des. Eng. 2016, 1, 202–207.
- You, S.S.; Cai, Q.; Müllen, K.; Yang, W.T.; Yin, M.Z. pH-sensitive unimolecular fluorescent polymeric micelles: From volume phase transition to optical response. Chem. Commun. 2014, 50, 823–825.
- Gao, B.X.; Li, H.X.; Liu, H.M.; Zhang, L.C.; Bai, Q.Q.; Ba, X.W. Water-soluble and fluorescent dendritic perylene bisimides for live-cell imaging. Chem. Commun. 2011, 47, 3894–3896.
- Ma, Y.; Zhang, F.; Zhang, J.; Jiang, T.; Li, X.; Wu, J.; Ren, H. A water-soluble fluorescent pH probe based on perylene dyes and its application to cell imaging. Luminescence 2016, 31, 102–107.
- Georgiev, N.I.; Said, A.I.; Toshkova, R.A.; Tzoneva, R.D.; Bojinov, V.B. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dyes Pigm. 2019, 160, 28–36.
- Aigner, D.; Dmitriev, R.I.; Borisov, S.M.; Papkovsky, D.B.; Klimant, I. pH-sensitive perylene bisimide probes for live cell fluorescence lifetime imaging. J. Mater. Chem. B 2014, 2, 6792–6801.
- Pacheco-Linan, P.J.; Moral, M.; Nueda, M.L.; Cruz-Sanchez, R.; Fernandez-Sainz, J.; Garzon-Ruiz, A.; Bravo, I.; Melguizo, M.; Laborda, J.; Albaladejo, J. Study on the pH dependence of the photophysical properties of a functionalized perylene bisimide and its potential applications as a fluorescence lifetime based pH probe. J. Phys. Chem. C 2017, 121, 24786–24797.
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and synthesis of novel fluorescence sensing perylene diimides based on photoinducedelectron transfer. Dyes Pigm. 2011, 91, 332–339.
- Daffy, L.M.; Silva, A.P.D.; Gunaratne, H.Q.N.; Huber, C.; Lynch, P.L.M.; Werner, T.; Wolfbeis, O.S. Arenedicarboximide building blocks for fluorescent photoinduced electron transfer pH sensors applicable with different media and communication wavelengths. Chem. Eur. J. 1998, 4, 1810–1815.
- Wu, J.H.; Peng, M.; Mu, M.X.; Li, J.; Yin, M.Z. Perylene diimide supramolecular aggregates: Constructions and sensing applications. Supramol. Mater. 2023, 2, 100031.
- Zhang, L.; Zhang, Y.F.; Han, Y.F. A perylene diimide-based fluorescent probe for the selective detection of hypochlorite in living cells. Mater. Chem. Front. 2022, 6, 2266–2273.
- Ma, L.; Gao, W.J.; Han, X.; Qu, F.L.; Xia, L.; Kong, R.M. A label-free and fluorescence turn-on assay for sensitive detection of hyaluronidase based on hyaluronan-induced perylene self-assembly. New. J. Chem. 2019, 43, 3383–3389.
- Kar, M.; Anas, M.; Banerjee, P.; Singh, A.; Sen, P.; Mandal, T.K. Amphiphilic perylene bisimide–polymer conjugates by cysteine-based orthogonal strategy: Vesicular aggregation, DNA binding, and cell imaging. ACS Appl. Polym. Mater. 2022, 4, 3697–3710.
- Zhao, Z.N.; Xu, N.; Wang, Y.; Ling, G.X.; Zhang, P. Perylene diimide-based treatment and diagnosis of diseases. J. Mater. Chem. B 2021, 9, 8937–8950.
