Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Alfred Zheng and Version 1 by Feng He.
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2)-KEAP1 system is the master regulator of cellular redox and metabolic homeostasis. NRF2 has Janus-like roles in carcinogenesis and cancer development. Short-term NRF2 activation suppresses tissue injury, inflammation, and cancer initiation.
- NRF2
- metabolism
- unfolded protein response
- Cancer
- Stress response
1. Introduction
All living organisms communicate with the environment and the environment poses constant threats to disrupt cell functions and shape its fate. Notably, cancer cells often display high levels of cellular GSH and ROS, in particular chemoresistant cancer cells, in which higher ROS levels activate antioxidant defense mechanisms, including nuclear factor erythroid 2 (NF-E2)-related factor 2 (NRF2), for the development of chemoresistance by reprogramming metabolism and alleviating drug-mediated oxidative stress that normally leads chemosensitive cancer cells to death [1].
2. NRF2 Functions in Carcinogenesis and Cancer Development
2.1. NRF2 Regulates Glutathione Metabolism and Antioxidant Defense in Tissue Injury and Tumorigenesis
O2 supply and demand imbalances cause ROS, and ROS induce the activation of NRF2 that in turn regulates cellular redox balance through the induction of professional enzymes dedicated to preventing the build-up of intracellular ROS, including the enzymes in phase I, II, and III of the drug detoxification reaction and elimination of pro-oxidants to maintain cellular homeostasis (Figure 1) [2]. Glutathione is a major antioxidant and is made from three amino acids: glycine, L-cysteine, and L-glutamate. The intracellular availability of Cys is determined by glutamate–cystine antiporter (xCT), encoded by solute carrier family 7 member 11 (SLC7A11), which exports glutamate in exchange for cystine uptake [3][4]. xCT expression is regulated by NRF2 [5]. NRF2 regulates glutathione metabolism through the induction of enzymes in glutathione synthesis, reduction, and redox cycling enzymes. Glutamate-cysteine ligase catalytic (GCLC) and modulator (GCLM) subunits, as well as glutathione synthetase (GSS), are the three NRF2 targets involved in the GSH synthesis and NRF2 stimulates the expression of GCLC, GCLM, and GSS (Figure 1) [2][4][5]. NRF2 induces the expression of GR, GPx2, GPx4, SOD1, CAT, and several glutathione S-transferases, which are responsible for glutathione utilization and redox cycling [2]. In addition, ROS can oxidize the Cys of proteins to the sulfenic form, resulting in structural changes of the proteins that alter their functions. The sulfenic form of Cys can be reduced to thiolate anions by the disulfide reductases thioredoxin (TRX) and glutaredoxin (GRX) to return the protein function to its original state [6]. TRX and GRX are direct targets of NRF2 [7]. By decreasing oxidative stress, NRF2 can prevent tissue and cell damage, thereby decreasing inflammation. Nfe2l2-deficient mice are also highly susceptible to drug-induced liver injury, alcoholic liver disease, and non-alcoholic fatty liver disease [8]. Compare with wild-type mice, Nfe2l2-null mice displayed more severe lung inflammation and damage, contributing to the pathogenesis of emphysema, upon exposure to cigarette smoke [9]. In addition, in a hyperoxia-induced acute lung injury (ALI) model, NRF2-knockout mice exhibited persistent cellular injury, impaired alveolar and endothelial cell regeneration, and persistent cellular infiltration by macrophages and lymphocytes, and this hyperoxia-induced damage was rescued by glutathione supplementation [10]. Considering the protective roles of NRF2 in tissue injury and repair, Nfe2l2−/− mice are more susceptible to chemical- and radiation-induced tumorigenesis, and NRF2 activators were reported to reduce the burdens of several cancers, including liver cancer [11][12], colon cancer [13], breast cancer [14], prostate cancer [15], and bladder cancer [16]. Therefore, as a master regulator of stress response against oxidative and toxic insults, NRF2 activation suppresses tissue injury, tumor-promoting inflammation, and cancer initiation.
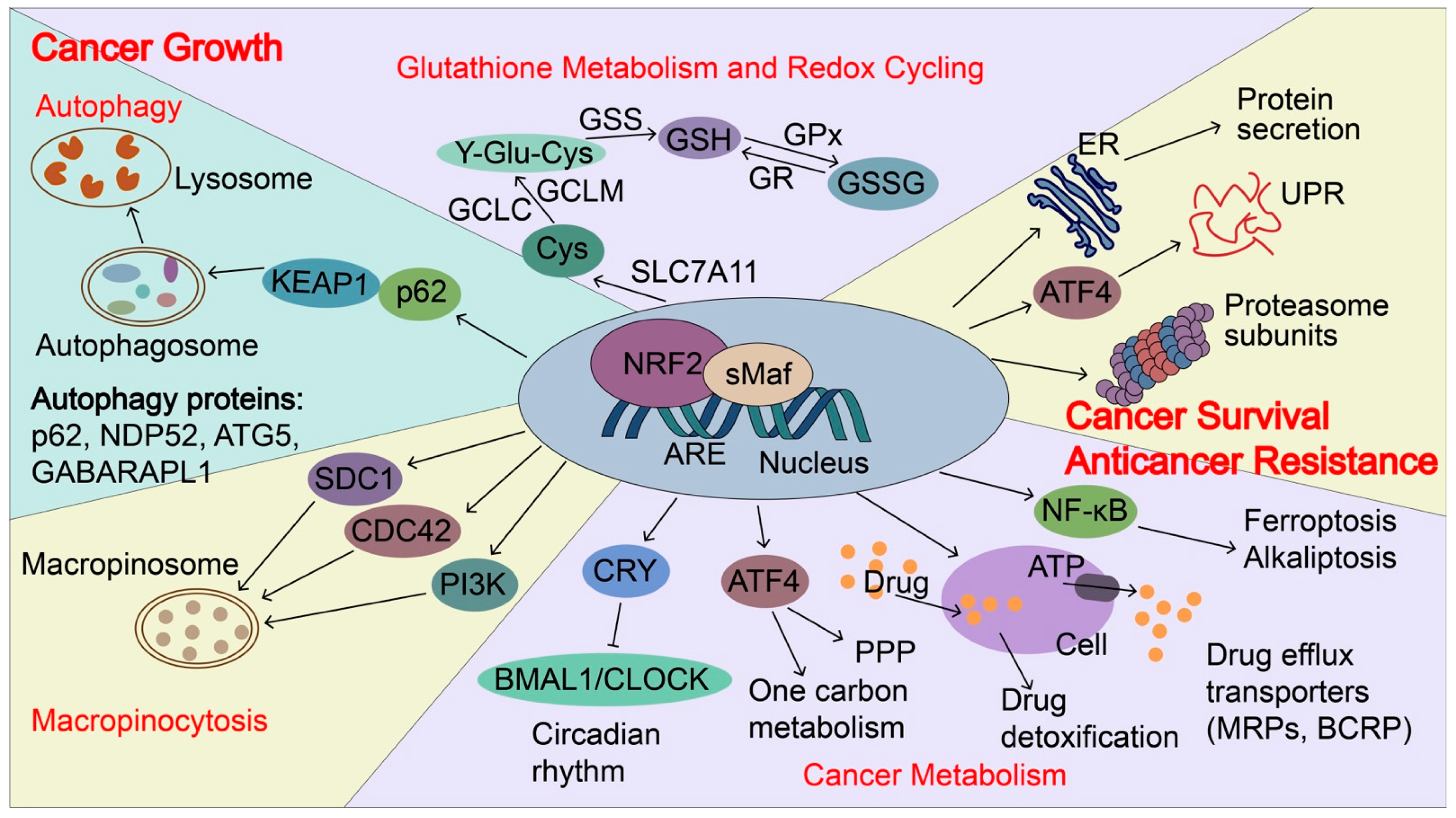
Figure 1. NRF2-induced targets regulate cancer growth, metastasis, and cancer drug resistance. In cancers, NRF2 signaling is aberrantly activated and NRF2 induces p62 expression and autophagy. p62 can directly interact with KEAP1, which causes NRF2 accumulation and KEAP1 degradation via the autophagy-related pathway. NRF2 transactivates the expression of genes encoding antioxidant proteins and drug-metabolizing enzymes, including glutathione metabolism and redox recycling. For example, NRF2 regulates the expression of SLC7A11 (xCT), Glutamate--cysteine ligase (GCLC and GCLM), and glutathione synthetase (GSS), all of which contribute to the elevation of reduced GSH levels. In addition, NRF2 regulates genes involved in autophagy, cancer metabolism, and macropinocytosis to support the nutrients demands for the rapid growth of cancer cells, including p62, antigen nuclear dot 52 kDa protein (NDP52), Autophagy protein 5 (ATG5), Gamma-aminobutyric acid receptor-associated protein-like 1 (GABARPL1), Syndecan-1 (SDC1), Cell division control protein 42 homolog (CDC42) and Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PIK3CG). Furthermore, NRF2 regulates unfolded protein response (UPR), proteostasis, cancer metabolism, and drug detoxification, which confer cancer metastasis and anticancer resistance. NRF2 transactivates the expression of genes encoding proteasome subunits, drug efflux transporters, multidrug resistance-associated proteins (MRPs/ABCCs), and breast cancer resistance proteins (BCRP/ABCG2). NRF2 also increases ATF4 expression and coordinates with ATF4 to regulate cancer metabolism, UPR, and protein secretion, as well as to maintain the state of the drug-tolerant persister cells. Furthermore, NRF2 represses CLOCK/BMAL1-regulated circadian rhythm by inducing the expression of CRY2 and integrating cellular redox signals and metabolism to promote tumorigenesis, cancer growth, and drug resistance. NDP52, antigen nuclear dot 52 kDa protein; ATG5, autophagy protein 5; GABARPL1, gamma-aminobutyric acid receptor-associated protein-like 1; SDC1, syndecan-1; CDC42, cell division control protein 42 homologs; PIK3CG, phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform; BMAL1: brain and muscle ARNT-like protein1; CLOCK: Circadian locomotor output cycles kaput; CRY2: Cryptochrome 2; GSS, glutathione synthetase; MRPs, multidrug resistance-associated proteins; BCRP, breast cancer resistance protein; SLC7A11(xCT), Solute carrier family 7 and member 11 (Cystine/glutamate transporter); GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modulator.
NRF2-activating mutations and loss of function mutations in KEAP1 and Cul3 that prevent effective NRF2 repression frequently occur in many cancers, such as liver cancer [17], lung cancer [18], ovarian cancer [19], kidney cancer [20], and breast cancer [21], resulting in constitutive activation of NRF2 signaling in cancer cells. All NRF2 mutations are located within the DLG (43%) and ETGE (57%) motifs, which are critical sites for the binding of NRF2 to KEAP1 [2][22]. About 19% of patients with lung cancer harbor somatic mutations in KEAP1, the third most commonly mutated gene behind the tumor-suppressor TP53 (46%) and KRAS (32%) oncogene [22][23]. Those mutations leading to NRF2 activation support the broad cancer-promoting roles of NRF2. Recent studies have shown that NRF2 has broader functions in regulating cancer progression.
2.2. NRF2 Regulates Autophagy, Cancer Metabolism, and Macropinocytosis for Cancer Growth
NRF2 regulates autophagy, cancer metabolism, and macropinocytosis to support the nutrient demands for the rapid growth of cancer cells. Autophagy is a vital process in which the body’s cells “clean out” any unnecessary or damaged components to allow intracellular nutrient recycling, especially under starvation conditions. Due to limited nutrient supply, cancer cells often elevate autophagy and depend on autophagy-mediated scavenging and recycling of intracellular macromolecules to maintain survival and growth [24][25]. NRF2 induces the expression of autophagy genes, including SQSTM1/p62, calcium-binding and coiled-coil domain-containing protein 2 (CALCOCO2/NDP52), autophagy protein 5 (ATG5), and gamma-aminobutyric acid receptor-associated protein-like 1 (GABARAPL1) to enhance autophagy [26]. In addition, NRF2 stimulates aerobic glycolysis, pentose phosphate pathway (PPP), de novo purine biosynthesis pathway, and amino acid and one-carbon metabolism to support cancer proliferation. The NRF2-induced genes to reprogram cancer metabolism have been extensively reviewed elsewhere [2][27]. In addition to the autophagy that supports intracellular nutrient recycling, a recent study showed that NRF2 mediates transcription of genes encoding the macropinocytosis pathway components, surface-localized syndecan 1 (SDC1), Na+/H+ exchanger 1 (NHE1), CDC42, and PIK3CG that induces an alternative route for tumors to scavenge nutrients from extracellular sources [28]. In pancreatic ductal adenocarcinoma (PDAC), NRF2 regulates collagenolysis and enables desmoplastic cancers to escape nutrient limitation, thus influencing patient survival [29]. Therefore, NRF2 provides various pathways for nutrient support to cancer cells and enables the growth advantages of cancer cells. This will make us reconsider the anti-cancer therapy based on blocking cancer nutrient supply, especially in NRF2 highly expressed cancers or cancers with super-activated NRF2 mutations. These NRF2-mediated nutrient support pathways could be promising anticancer targets.
2.3. NRF2 Regulates Unfolded Protein Response (UPR) and Proteostasis for Cancer Metastasis and Resistance to Anticancer Therapy
UPR is the mechanism by which cells control endoplasmic reticulum (ER) protein synthesis, folding, modification, and transport of secretory and organelle-bound proteins, as well as their degradation. ER stress during cancer growth, stress-rich microenvironments, such as low pH, hypoxia, nutrition deprivation, and metabolic stress, can induce ROS formation and accumulation of misfolded proteins that lead to ER stress and UPR via activation of three signaling arms coordinated by IRE1-XBP1, PERK-eIF2a-ATF4, and ATF6. During metastasis, metastasizing cancer cells have to be able to survive from migration through the stroma, intravasation through the endothelium into the blood or lymphatic vessels, circulation in the vessels, and subsequently extravasation through the endothelium, and colonization at distant tissues. All of these processes exhibit various levels of mechanical forces, including fluid shear stress, hydrostatic pressure, and tension and compression forces, which trigger stress responses. UPR is upregulated in cancers and UPR coordinates with NRF2 to sustain cancer survival, proliferation, and metastasis [27]. ER stress and UPR can activate NRF2 by PERK-mediated phosphorylation of NRF2 [30]. Upon targeted and conventional cancer therapies, cancer cells often induce stress response to survive the cancer treatment and generate drug tolerance via heme-regulated inhibitor (HRI) kinase-ATF4 signaling [31]. Chronic sublethal stress is a major feature of drug-tolerant persister cells. In addition, high NRF2 activity regulates drug detoxification through the induction of antioxidant proteins and phase two metabolizing enzymes. Drug efflux transporters, such as multidrug resistance-associated proteins (MRPs/ABCCs) and breast cancer resistance protein (BCRP/ABCG2), which facilitate xenobiotic detoxification by preventing the intracellular accumulation of foreign substances, contain the functional AREs in their promoter or enhancer regions and they are direct targets of NRF2 [32]. NRF2 activates the transcription of ATF4 which regulates amino-acid metabolism and anticancer drug resistance [33]. NRF2 activation in mice liver induces the expression of genes involved in the UPR and protein secretion [5].
ER stress and UPR share an intimate connection with proteostasis. NRF2 regulates the activity of the proteasome. NRF2 regulates the expression of the 20S proteasome subunits PSMA1, PSMB3, and PSMB6 and 19S proteasome subunits PSMC1, PSMC3, and PSMD14, as well as a proteasome chaperone-proteasome maturation protein (POMP) [34]. Not all of them harbor the conserved ARE motifs, suggesting that NRF2 may coordinate with other transcription factors to induce the transcription of proteasomes subunits. Proteotoxic stress can activate NRF2 by inactivating ARE-transcriptional repressor BACH1 [35]. Elevated NRF2 activation in cancers treated with proteasome inhibitor bortezomib contributes to bortezomib resistance [35]. In summary, NRF2 increases UPR and proteasome activity, together with the expression of anti-oxidant and drug detoxification enzymes, contributing to stress adaptation.
2.4. NRF2 Regulates Circadian Rhythm to Promote Tumorigenesis and Cancer Growth
The circadian rhythm is a natural, internal process that regulates the sleep–wake cycle and other human activities in a manner of roughly every 24-h period of light and dark on Earth. It is driven by the circadian clock, an evolutionarily conserved timekeeping system for numerous biological rhythms that allow organisms to anticipate and adapt their behavior and physiology to predictable changes in their environment [36]. Numerous studies indicate that circadian rhythm disruptions (e.g., jet lag, shift work, sleep disruption, and exposure to light at night) are associated with increased cancer risk [37][38][39][40] and World Health Organization designated circadian disruption as a likely carcinogen [36]. The circadian clock is composed of a core transcription-translation feedback loop, in which transcriptional factors aryl hydrocarbon receptor nuclear translocator-like (ARNTL/BMAL1) and circadian locomotor output cycles kaput (CLOCK) activate the transcription of their own repressors, period 1/2/3 (PER1/2/3) and cryptochrome 1/2 (CRY1/2) that in turn represses CLOCK/BMAL1-regulated E-box transcription [37]. Cellular redox potential, metabolism, and circadian rhythms are closely linked. NRF2 is an important bridge between the molecular clock and metabolism. Wible et al. showed that chemical activation of NRF2 or genetic NRF2 activation induces CRY2 expression by binding to the specific enhancer regions of the CRY2 gene, resulting in the repression of CLOCK/BMAL1-regulated E-box transcription and alteration of circadian rhythms [41]. Nfe2l2-deficient mouse fibroblasts, hepatocytes and liver also altered rhythmicity. These data support that NRF2 links metabolism signals to the ticking of the circadian clock. In addition, NRF2 activation at a circadian time corresponding to the peak generation of endogenous oxidative signals resulted in NRF2-dependent reinforcement of circadian amplitude, suggesting that NRF2 amount and/or timing of expression are important to timekeeping in cells. Furthermore, NRF2 itself is also transcriptionally upregulated by BAML1 [42][43]. Therefore, NRF2 and the circadian clock comprise an interlocking negative feedback loop that integrates cellular redox signals and metabolism to promote tumorigenesis, cancer growth, and drug resistance. Small molecules targeting the circadian signaling pathways become a new therapeutic method for cancer treatment due to their close relationships with cancer [37]. Therefore, NRF2-targeted small molecules and circadian modifying agents could be combined to treat cancer with better efficiency in the future.
References
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365.
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777.
- Sayin, V.I.; LeBoeuf, S.E.; Singh, S.X.; Davidson, S.M.; Biancur, D.; Guzelhan, B.S.; Alvarez, S.W.; Wu, W.L.; Karakousi, T.R.; Zavitsanou, A.M.; et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. Elife 2017, 6, e28083.
- Fu, J.; Xiong, Z.; Huang, C.; Li, J.; Yang, W.; Han, Y.; Paiboonrungruan, C.; Major, M.B.; Chen, K.N.; Kang, X.; et al. Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus. J. Biol. Chem. 2019, 294, 327–340.
- He, F.; Antonucci, L.; Yamachika, S.; Zhang, Z.; Taniguchi, K.; Umemura, A.; Hatzivassiliou, G.; Roose-Girma, M.; Reina-Campos, M.; Duran, A.; et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J. Hepatol. 2020, 72, 1182–1195.
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462.
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151.
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079–13087.
- Iizuka, T.; Ishii, Y.; Itoh, K.; Kiwamoto, T.; Kimura, T.; Matsuno, Y.; Morishima, Y.; Hegab, A.E.; Homma, S.; Nomura, A.; et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 2005, 10, 1113–1125.
- Reddy, N.M.; Kleeberger, S.R.; Kensler, T.W.; Yamamoto, M.; Hassoun, P.M.; Reddy, S.P. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J. Immunol. 2009, 182, 7264–7271.
- Johnson, N.M.; Egner, P.A.; Baxter, V.K.; Sporn, M.B.; Wible, R.S.; Sutter, T.R.; Groopman, J.D.; Kensler, T.W.; Roebuck, B.D. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev. Res. 2014, 7, 658–665.
- Kitamura, Y.; Umemura, T.; Kanki, K.; Kodama, Y.; Kitamoto, S.; Saito, K.; Itoh, K.; Yamamoto, M.; Masegi, T.; Nishikawa, A.; et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazoquinoline. Cancer Sci. 2007, 98, 19–24.
- Long, M.; Tao, S.; Rojo de la Vega, M.; Jiang, T.; Wen, Q.; Park, S.L.; Zhang, D.D.; Wondrak, G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev. Res. 2015, 8, 444–454.
- Kim, E.H.; Deng, C.; Sporn, M.B.; Royce, D.B.; Risingsong, R.; Williams, C.R.; Liby, K.T. CDDO-methyl ester delays breast cancer development in BRCA1-mutated mice. Cancer Prev. Res. 2012, 5, 89–97.
- Frohlich, D.A.; McCabe, M.T.; Arnold, R.S.; Day, M.L. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene 2008, 27, 4353–4362.
- Iida, K.; Itoh, K.; Maher, J.M.; Kumagai, Y.; Oyasu, R.; Mori, Y.; Shimazui, T.; Akaza, H.; Yamamoto, M. Nrf2 and p53 cooperatively protect against BBN-induced urinary bladder carcinogenesis. Carcinogenesis 2007, 28, 2398–2403.
- Eichenmuller, M.; Trippel, F.; Kreuder, M.; Beck, A.; Schwarzmayr, T.; Haberle, B.; Cairo, S.; Leuschner, I.; von Schweinitz, D.; Strom, T.M.; et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J. Hepatol. 2014, 61, 1312–1320.
- Padmanabhan, B.; Tong, K.I.; Ohta, T.; Nakamura, Y.; Scharlock, M.; Ohtsuji, M.; Kang, M.I.; Kobayashi, A.; Yokoyama, S.; Yamamoto, M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell 2006, 21, 689–700.
- Konstantinopoulos, P.A.; Spentzos, D.; Fountzilas, E.; Francoeur, N.; Sanisetty, S.; Grammatikos, A.P.; Hecht, J.L.; Cannistra, S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011, 71, 5081–5089.
- Ooi, A.; Dykema, K.; Ansari, A.; Petillo, D.; Snider, J.; Kahnoski, R.; Anema, J.; Craig, D.; Carpten, J.; Teh, B.T.; et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res. 2013, 73, 2044–2051.
- Nioi, P.; Nguyen, T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem. Biophys. Res. Commun. 2007, 362, 816–821.
- Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; Murray, B.A.; et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016, 48, 607–616.
- Cancer Genome Atlas Research, N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550.
- Umemura, A.; He, F.; Taniguchi, K.; Nakagawa, H.; Yamachika, S.; Font-Burgada, J.; Zhong, Z.; Subramaniam, S.; Raghunandan, S.; Duran, A.; et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell 2016, 29, 935–948.
- Taniguchi, K.; Yamachika, S.; He, F.; Karin, M. p62/SQSTM1-Dr. Jekyll and Mr. Hyde that prevents oxidative stress but promotes liver cancer. FEBS Lett. 2016, 590, 2375–2397.
- Pajares, M.; Jimenez-Moreno, N.; Garcia-Yague, A.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rabano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916.
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43.
- Su, H.; Yang, F.; Fu, R.; Li, X.; French, R.; Mose, E.; Pu, X.; Trinh, B.; Kumar, A.; Liu, J.; et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 2021, 39, 678–693 e611.
- Su, H.; Yang, F.; Fu, R.; Trinh, B.; Sun, N.; Liu, J.; Kumar, A.; Baglieri, J.; Siruno, J.; Le, M.; et al. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature 2022, 610, 366–372.
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 2003, 23, 7198–7209.
- Kalkavan, H.; Chen, M.J.; Crawford, J.C.; Quarato, G.; Fitzgerald, P.; Tait, S.W.G.; Goding, C.R.; Green, D.R. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 2022, 185, 3356–3374.e3322.
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Effect of Graded Nrf2 Activation on Phase-I and -II Drug Metabolizing Enzymes and Transporters in Mouse Liver. PLoS ONE 2012, 7, e39006.
- DeNicola, G.M.; Chen, P.H.; Mullarky, E.; Sudderth, J.A.; Hu, Z.; Wu, D.; Tang, H.; Xie, Y.; Asara, J.M.; Huffman, K.E.; et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat. Genet. 2015, 47, 1475–1481.
- Kwak, M.K.; Wakabayashi, N.; Greenlaw, J.L.; Yamamoto, M.; Kensler, T.W. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol. Cell Biol. 2003, 23, 8786–8794.
- Rushworth, S.A.; Bowles, K.M.; MacEwan, D.J. High basal nuclear levels of Nrf2 in acute myeloid leukemia reduces sensitivity to proteasome inhibitors. Cancer Res. 2011, 71, 1999–2009.
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814.
- Wang, Y.; Guo, H.; He, F. Circadian disruption: From mouse models to molecular mechanisms and cancer therapeutic targets. Cancer Metastasis Rev. 2022.
- Wendeu-Foyet, M.G.; Menegaux, F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol. Biomark. Prev. 2017, 26, 985–991.
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307.
- Kettner, N.M.; Voicu, H.; Finegold, M.J.; Coarfa, C.; Sreekumar, A.; Putluri, N.; Katchy, C.A.; Lee, C.; Moore, D.D.; Fu, L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 2016, 30, 909–924.
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 2018, 7, e31656.
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468.
- Sun, Q.; Zeng, C.; Du, L.; Dong, C. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp. Ther. Med. 2021, 21, 190.
More
