Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Bin Li.
Nickerson and Habrl [22] extracted nanomaterials named nanocellulose (NC) from cotton linters by using sulfuric-acid hydrolysis in 1947. Since then, various physical and chemical properties of NC, such as its low weight, low cost, high strength, stiffness, and non-toxic properties have been comprehensively investigated in both academia and industry.
- nanocellulose
- surface functionality
- cellulose
1. Introduction
Environmental awareness has focused significant attention on the better utilization of sustainable natural polymers, such as cellulose, chitin, and starch [1,2][1][2]. Cellulose, which has a polysaccharide structure, is abundantly available across the Earth, and it is present in woody and non-woody plants, as well as some sea animals, such as tunicates [3,4][3][4]. Cellulosic fibers were used as lumber, Chinese Xuan paper, textiles, and cordages for thousands of years [5]. The French chemist, Anselme Payen, first isolated cellulose from plants in 1839, and Staudinger later determined the polymeric structure of cellulose in the 1920s [6]. In nature, all plants, even tall trees (some of which are over 120 m in height), are supported by the hierarchical structures of wood-cell walls, which consist of the primary cell, the secondary wall, and the lumens (Figure 1a) [7]. In particular, the secondary wall (thickness ≈ 4 µm) can be further subdivided into three concentric layers, S1–S3, and the lumen forms the hollow center [8]. Furthermore, the S2 layer holds most of the cellulose microfibrils (MFs), with diameters in the range of 10–30 nm and lengths reaching more than 2 µm [9,10,11][9][10][11]. The MFs can be further divided into tens of elementary fibrils (EFs), with diameters ranging from 3 to 5 nm and lengths reaching more than 1 µm [12]. Furthermore, the fact that EFs are surrounded by hemicellulose and lignin and hierarchically packed together endows MFs with extraordinary structural stability. The EFs are formed from cellulose-molecule chains, which are considered fundamental parts of the wood cell wall. Each cellulose molecule has abundant hydroxyl (–OH) groups that can form intra-hydrogen bonds, thus stabilizing the nanofibers through inter-hydrogen bonds and promoting parallel chain stacking [13]. Hemicellulose constitutes 20–30% of the wood dry mass. Compared to cellulose, hemicellulose has a shorter crosslinked chain structure (DP ≈ 200) (Figure 1b) [14]. Additionally, hemicellulose is made of several monomers (e.g., glucose, xylose, and arabinose), whereas cellulose is comprised of glucose monomers only [15]. Moreover, compared to cellulose, the remarkable aspect of hemicellulose is its diversity in terms of the types of side group, the units of composition, the molecular weights, and the branching sites [16]. The diversity of hemicellulose present challenges when examining the relationship between its intrinsic heterogeneity and the properties of its final products. Furthermore, hemicellulose combines with cellulose, via hydrogen bonds, and lignin, via hydrogen bonds and ether bonds, strengthening the stability of the cell wall [17]. Lignin is a complex phenolic polymer of aromatic compounds with three main aromatic subunits (p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol), which are mainly linked together by C-C bonds and ether bonds, leading to a complex and irregular macromolecular structure (Figure 1b) [18,19][18][19]. Lignin is hydrophobic by nature, and has a much higher degree of crosslinking than hemicellulose, which contributes to the elasticity and mechanical strength of the plant [20].
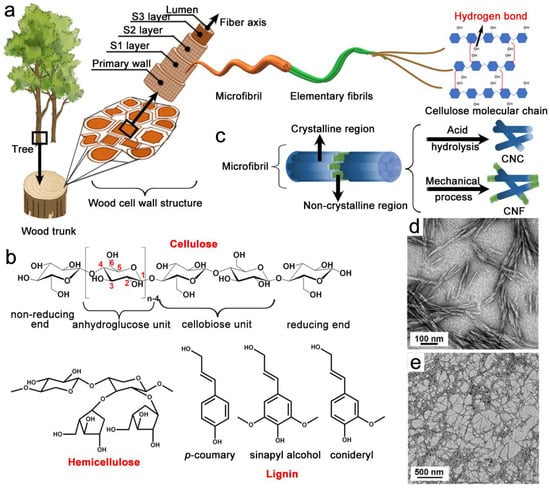
Figure 1. Hierarchical structure of cellulose in plants (a); molecular structures of cellulose, hemicellulose, and lignin (the three primary components of the plant cell wall) (b); schematic illustration of the preparation of CNC and CNF (c); TEM images of CNC (d) and CNF (e) [21].
Nickerson and Habrl [22] extracted nanomaterials named nanocellulose (NC) from cotton linters by using sulfuric-acid hydrolysis in 1947. Since then, various physical and chemical properties of NC, such as its low weight, low cost, high strength, stiffness, and non-toxic properties have been comprehensively investigated in both academia and industry. Due to the presence of abundant surface hydroxyl groups, NC can be easily functionalized by amination, silanization, carboxylation, or esterification to obtain different cellulose derivatives. Moreover, some small molecular substances, such as dopamine, tannic acid, acrylate, and acrylamide, can be coated or grafted on the surface of cellulose to obtain functional NC. The functionality of NC is of vital importance for its final applications. However, there is a comprehensive summary focused on the introduction and definition of NC substrates, the chemical modification routes applied so far for the functionalization of NC, and NC-based functional materials is still lacking. Moreover, it still remains a challenge to modify NC simply and efficiently without destroying the original morphology and crystalline structure. Herein, thies reviewe contents summarizes the conceptual methods applied to, the current status of chemical modification routes for, and the morphologies and reactions of different functionalized NCs, including TEMPO-mediated oxidation, periodate oxidation, esterification, etherification, silanization, surface coating, and grafting. Most importantly, the functionality of NC is strongly linked to its application performance. Therefore, we correspondingly introduce NC-based functional materials, such as paper-based devices, antimicrobial packaging, pollutant absorption, conducting polymer hydrogels, wearable sensor, and flexible electrodes have been introduced accordingly.
2. Structures and Characteristics of Cellulose and Nanocellulose
2.1. Cellulose
Driven by the awareness of the better utilization of sustainable natural polymers, cellulose could serve as a promising alternative material for petroleum-based materials due to its virtues of abundance, sustainability, degradability, and biocompatibility [1]. Cellulose is abundantly available from plants and other sources, such as trees, bamboo, hemp, cotton, agricultural crops, bacteria, tunicates, and algae [23,24,25][23][24][25]. Moreover, as a green polymer, cellulose is suitable for utilization in sustainable materials engineering. From a top-down perspective, lignin and hemicellulose need to be dissolved/depolymerized from plants, followed by processing to obtain cellulose for various end uses [26]. Cellulose with repeated cellobiose units is the most abundant natural polysaccharide. As shown in Figure 1b, the cellobiose unit is assembled by two anhydroglucose rings rotated by 180° relative to each other and connected by β-1,4 glycosidic bond [24]. The general formula of cellulose is (C6H10O5)n, where n is the degree of polymerization (DP), depending on the cellulose’s source material and the preparation approach [14]. Each cellulose chain has a hemiacetal group and chemically reducing functionality, and the other end possesses a pendant hydroxyl group, the nominal non-reducing end. Moreover, bacterial cellulose (BC, a kind of microbial cellulose), with high levels of water retention and a specific surface area, is typically synthesized by Gram-negative or Gram-positive bacteria (including Acetobacter xylinum, Acetobacter, Alcaligenes, Pseudomonas, and others) [27,28][27][28]. The DP of BC varies from 2000 to 6000, with diameters in the range of 10–50 nm and lengths in the range of 100–1000 nm [29,30][29][30]. Bacterial cellulose has been widely used in food additives, bio-medical sectors, and bio-based nanocomposites due to its high purity, distinct physicochemical characteristics, and biodegradability [31]. Different forms of BC can be produced by changing its mode of fermentation. Under agitation or stirring, sharp or irregular sphere-like BC particles are formed, while BC materials with three-dimensional interconnected structures can be produced in static conditions.2.2. Nanocellulose
As is widely known, various chemical pulping and bleaching reactions are adopted to fully remove lignin and partially remove hemicelluloses to obtain cellulose pulp [7]. Next, mechanical treatments (e.g., high-pressure homogenization, high-intensity ultrasonication, or ultrafine grinding) or inorganic acid hydrolysis (e.g., sulfuric, hydrochloric, maleic, or phosphoric acid) are used to refine the cellulose pulp (Figure 1c) [32,33,34,35,36][32][33][34][35][36]. Subsequently, NC derived from lignocellulose is divided into two generic forms, cellulose nanofibril (CNF) and cellulose nanocrystal (CNC) [37]. In general, CNF is a flexible, fiber-like, and semicrystalline cellulose nanomaterial with diameters of less than 100 nm, typically ranging from 3 to 50 nm, and lengths reaching over 1000 nm (Figure 1d) [38]. Cellulose nanocrystal is a rigid rod-like cellulose nanomaterial with a diameter of 10–30 nm and a length of 5–200 nm, which is mostly crystalline in nature (Figure 1e) [39]. The properties (length, diameter, aspect ratio, modulus, strength, and specific surface area) of NC generally depend on the cellulose source, as well as the preparation method and conditions (such as the concentration of chemicals, the treatment temperature, and the treatment time). It is possible to produce CNFs with a high aspect ratio (>100), excellent tensile modulus, and entangled morphologies, by using mechanical nano-fibrillation methods such as homogenization, ultrasonication, microfluidization, and grinding. In particular, high-pressure homogenization is the most commonly used method for producing CNFs with high quality, which can break down cellulose-pulp fibers and release nanofibrils through various forces, such as rapid changes in high pressure, strong shear, high speed, and turbulence [40,41][40][41]. However, some issues need to be carefully addressed when using this method, particularly its large energy consumption and relatively low production yield. Thus, some pretreatments were invented recently to address these drawbacks, such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation, formic acid (FA) hydrolysis, ionic liquid (IL) treatment, enzymatic hydrolysis, carboxymethylation, and others [42,43][42][43]. These pretreatments contribute to the swelling of the fiber wall, which effectively loosens the interfibrillar hydrogen bonds, which supports the subsequent mechanical treatment. It should be pointed out that some pretreatment methods (such as TEMPO-mediated oxidation and FA hydrolysis) do not only reduce the energy consumption of the subsequent mechanical homogenization during NC production, but also simultaneously introduce some new functional groups (carboxyl and ester groups, accordingly) into NCs during pretreatment, which endow the NCs with different surface properties, which are heavily linked to its application performance. Enzyme pretreatments, such as cellulase, hemicellulases, and ligninases, have been used to facilitate the production of CNF, which breaks down targeted bonds in pulp [44]. However, the efficiency and activity of enzymatic pretreatments depend on the enzyme dosage, reaction time, pH, and reaction temperature, which limit its application in the large-scale production of CNF. Additionally, green solvents (e.g., ILs, DES) have been extensively used in pretreatments in NC fibrillation because of their ability to loosen the cellulose network by disrupting hydrogen bonds [45]. Unlike CNF, CNC, which has a relatively low aspect ratio, is typically obtained through the strong inorganic acid hydrolysis of cellulosic pulp, which is used to remove disordered and paracrystalline regions in cellulose, such as sulfuric acid, phosphoric acid, hydrochloric acid, nitric acid, and hydrobromic acid [35,46,47][35][46][47]. Among these hydrolyzed acids, sulfuric acid has been widely used for the production of CNC, since CNC demonstrates exceptional dispersity in water, high hydrolytic efficiency, and simple and time-saving process [48]. Generally, the disordered regions (amorphous structure) are mostly degraded during acid hydrolysis due to the loose structure, while the crystalline structure of cellulose remains as CNC due to its stability [49]. However, the issues in the use of sulfuric acid for CNC production, such as the large usage of water, the harsh corrosion of the equipment used, and the relatively low production yield, need to be rigorously addressed [50]. Thus, some recoverable organic acids and solid acids, such as oxalic acid, maleic acid, FA, and phosphotungstic acid, have been used to prepare CNC through a sustainable and environmentally friendly process. For instance, oxalic acid was used to hydrolyze hardwood pulp to prepare CNC, and the highest yield of the CNC was around 25 wt% [51]. Furthermore, the incompletely hydrolyzed solid cellulosic residue was used as feedstock to produce CNF through homogenization. In recent years, FA, as a recyclable organic carboxylic acid, has been used to hydrolyze cellulose for producing CNC [21]. Compared with other inorganic strong acids, FA can be easily recovered and reused due to its lower boiling point (100.8 °C), which is less corrosive to equipment [52]. Additionally, FA can efficiently hydrolyze hemicellulose, remove lignin, and maintain cellulose; thus FA has been widely used to pretreat various cellulosic materials in the production of CNC. The modification of NC is of crucial importance to ensure the functionality of its various final applications. In general, chemical modifications, such as silanization, esterification, and etherification, occur in reaction of different chemicals with the hydroxyl groups of NC. In contrast, some chemical modifiers, such as dopamine, tannic acid, acrylates, and acrylamides, can also be coated/grafted on the surfaces of cellulose materials. These reactions are sensitive to water and are typically present at low concentrations, which increase the reagent consumption and make the whole process environmentally unfriendly and difficult to scale up. Various modification processes have been applied because different NCs have different features. For these reasons, it remains challenging to modify NC surfaces simply and efficiently.References
- Durairaj, A.; Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Cellulose Nanocrystals (Cnc)-Based Functional Materials for Supercapacitor Applications. Nanomaterials 2022, 12, 1828.
- Zhang, Y.; Deng, W.; Wu, M.; Liu, Z.; Yu, G.; Cui, Q.; Liu, C.; Fatehi, P.; Li, B. Robust, Scalable, and Cost-Effective Surface Carbonized Pulp Foam for Highly Efficient Solar Steam Generation. ACS Appl. Mater. Interfaces 2023, 15, 7414–7426.
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665.
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional Nanomaterials through Esterification of Cellulose: A Review of Chemistry and Application. Cellulose 2018, 25, 3703–3731.
- Habibi, Y. Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542.
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779.
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and Interface Engineering for Nanocellulosic Advanced Materials. Adv. Mater. 2021, 33, 2002264.
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523.
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent Paper: Fabrications, Properties, and Device Applications. Energy Environ. Sci. 2014, 7, 269–287.
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin Anode for Sodium-Ion Batteries Using Natural Wood Fiber as a Mechanical Buffer and Electrolyte Reservoir. Nano Lett. 2013, 13, 3093–3100.
- Fernandes, A.N.; Thomas, L.H.; Altaner, C.M.; Callow, P.; Forsyth, V.T.; Apperley, D.C.; Kennedy, C.J.; Jarvis, M.C. Nanostructure of Cellulose Microfibrils in Spruce Wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195–E1203.
- Chen, C.; Hu, L. Nanocellulose toward Advanced Energy Storage Devices: Structure and Electrochemistry. Accounts Chem. Res. 2018, 51, 3154–3165.
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer Nanofibrils: Structure, Modeling, Preparation, and Applications. Prog. Polym. Sci. 2018, 85, 1–56.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Whitney, S.E.; Gothard, M.G.; Mitchell, J.T.; Gidley, M.J. Roles of Cellulose and Xyloglucan in Determining the Mechanical Properties of Primary Plant Cell Walls. Plant Physiol. 1999, 121, 657–663.
- Gorshkova, T.A.; Kozlova, L.; Mikshina, P. Spatial Structure of Plant Cell Wall Polysaccharides and Its Functional Significance. Biochem.-Mosc. 2013, 78, 836–853.
- Pérez, J.; Muñoz-Dorado, J.; De La Rubia, T.; Martínez, J. Biodegradation and Biological Treatments of Cellulose, Hemicellulose and Lignin: An Overview. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2002, 5, 53–63.
- Yang, X.; Berthold, F.; Berglund, L.A. Preserving Cellulose Structure: Delignified Wood Fibers for Paper Structures of High Strength and Transparency. Biomacromolecules 2018, 19, 3020–3029.
- Petridis, L.; Smith, J.C. Conformations of Low-Molecular-Weight Lignin Polymers in Water. Chemsuschem 2016, 9, 289–295.
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of Cellulose, Hemicellulose and Lignin on Thermochemical Conversion Characteristics of the Selected Biomass. Bioresour. Technol. 2012, 114, 663–669.
- Lv, D.; Du, H.; Che, X.; Wu, M.; Zhang, Y.; Liu, C.; Nie, S.; Zhang, X.; Li, B. Tailored and Integrated Production of Functional Cellulose Nanocrystals and Cellulose Nanofibrils Via Sustainable Formic Acid Hydrolysis: Kinetic Study and Characterization. ACS Sustain. Chem. Eng. 2019, 7, 9449–9463.
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind Eng Chem. Ind. Eng. Chem. 1947, 39, 1507–1512.
- Wyatt, S.E.; Kiss, J.Z. Plant Tropisms: From Darwin to the International Space Station. Am. J. Bot. 2013, 100, 1191–1201.
- Ray, U.; Zhu, S.; Pang, Z.; Li, T. Mechanics Design in Cellulose-Enabled High-Performance Functional Materials. Adv. Mater. 2020, 33, 2002504.
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2020, 33, e2000619.
- Qin, Y.; Qiu, X.; Zhu, J. Understanding Longitudinal Wood Fiber Ultra-Structure for Producing Cellulose Nanofibrils Using Disk Milling with Diluted Acid Prehydrolysis. Sci. Rep. 2016, 6, 35602.
- Jonas, R.; Farah, L.F. Production and Application of Microbial Cellulose. Polym. Degrad. Stab. 1998, 59, 101–106.
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984.
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12.
- Petersen, N.; Gatenholm, P. Bacterial Cellulose-Based Materials and Medical Devices: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286.
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. Bioresources 2016, 11, 5641–5655.
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A Novel Approach for the Preparation of Nanocrystalline Cellulose by Using Phosphotungstic Acid. Carbohydr. Polym. 2014, 110, 415–422.
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and Characterization of Cellulose Nanowhiskers from Cotton Fibres by Controlled Microbial Hydrolysis. Carbohydr. Polym. 2011, 83, 122–129.
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile Extraction of Thermally Stable Cellulose Nanocrystals with a High Yield of 93% through Hydrochloric Acid Hydrolysis under Hydrothermal Conditions. J. Mater. Chem. A 2013, 1, 3938–3944.
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of Cellulose Nanocrystals Using Hydrobromic Acid and Click Reactions on Their Surface. J. Mater. Sci. 2011, 46, 7344–7355.
- Cheng, M.; Qin, Z.; Chen, Y.; Liu, J.; Ren, Z. Facile One-Step Extraction and Oxidative Carboxylation of Cellulose Nanocrystals through Hydrothermal Reaction by Using Mixed Inorganic Acids. Cellulose 2017, 24, 3243–3254.
- Zhang, Y.; Liu, C.; Wu, M.; Li, Z.; Li, B. Impact of the Incorporation of Nano-Sized Cellulose Formate on the End Quality of Polylactic Acid Composite Film. Nanomaterials 2021, 12, 1.
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and Characterization of Functional Cellulose Nanofibrils Via Formic Acid Hydrolysis Pretreatment and the Followed High-Pressure Homogenization. Ind. Crops Prod. 2016, 94, 736–745.
- Cai, J.; Zhang, L.; Zhou, J.; Qi, H.; Chen, H.; Kondo, T.; Chen, X.; Chu, B. Multifilament Fibers Based on Dissolution of Cellulose in Naoh/Urea Aqueous Solution: Structure and Properties. Adv. Mater. 2007, 19, 821–825.
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25.
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969.
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of Wood-Based Microfibrillated Cellulose and Nanofibrillated Cellulose, and Applications Relating to Papermaking: A Review. Cellulose 2016, 23, 93–123.
- Isogai, A.; Saito, T.; Fukuzumi, H. Tempo-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85.
- Liu, X.; Jiang, Y.; Wang, L.; Song, X.; Qin, C.; Wang, S. Tuning of Size and Properties of Cellulose Nanofibers Isolated from Sugarcane Bagasse by Endoglucanase-Assisted Mechanical Grinding. Ind. Crops Prod. 2020, 146, 112201.
- Li, K.; Choudhary, H.; Rogers, R.D. Ionic Liquids for Sustainable Processes: Liquid Metal Catalysis. Curr. Opin. Green Sustain. Chem. 2018, 11, 15–21.
- Espinosa, S.C.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of Thermally Stable Cellulose Nanocrystals by Phosphoric Acid Hydrolysis. Biomacromolecules 2013, 14, 1223–1230.
- Cao, Y.; Jiang, Y.; Song, Y.; Cao, S.; Miao, M.; Feng, X.; Fang, J.; Shi, L. Combined Bleaching and Hydrolysis for Isolation of Cellulose Nanofibrils from Waste Sackcloth. Carbohydr. Polym. 2015, 131, 152–158.
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346.
- de Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.D.S.; Belgacem, M.N. Production of Cellulose Nanocrystals from Sugarcane Bagasse Fibers and Pith. Ind. Crops Prod. 2016, 93, 48–57.
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068.
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly Thermal-Stable and Functional Cellulose Nanocrystals and Nanofibrils Produced Using Fully Recyclable Organic Acids. Green Chem. 2016, 18, 3835–3843.
- Gao, M.; Shang, Y.; Li, B.; Du, H. Sustainable Preparation of Cellulose Nanocrystals: State of the Art and Perspectives. Green Chem. 2022, 24, 9346–9372.
More
