You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Béla Dénes and Version 3 by Jessie Wu.
The members of Brucella spp. as Gram-negative bacteria are characterized by their sandwich-structured cell envelopes composed of the lipopolysaccharide (LPS)-covered bacterial outer membrane and the inner cytoplasmic cell membrane with a thin peptidoglycan layer between them in the periplasmic space.
- brucellosis
- serology
- false positive serologic results (FPSR)
- Gram-negative bacteria
- lipopolysaccharide (LPS)
- smooth (S) colony types
- rough (R) colony types
- cell-free DNA (cfDNA)
- next generation sequencing
1. Introduction
Human and animal brucellosis and its health and economic consequences have been known for millennia. The pathogens behind the diseases, the Brucella species, were first described by Bruce, along with the rapid development of the first serologic diagnostic probe detecting Brucella infection by Wright at the end of the 19th century [1][2][3]. Since then, the genus Brucella has expanded to more than 30 known species, including isolates from exotic hosts, such as cetaceans or the surface of human breast implants [4], demonstrating the physiological and genetic flexibility of the bacteria.
Besides the ability to exist in a wide variety of hosts, this flexibility provides environmental persistence (against extreme temperature, pH, and humidity) outside of any host [5], as well as the ability of intracellular localization within the host organism [6][7][8] for most Brucella species. By completing the picture with the mild toxicity of the Brucella endotoxin—three orders of magnitude lower than the respective E. coli molecule [9]—the difficulties relating to the recognition and diagnosis of brucellosis are subsequently compounded.
2. Issues with the Antigenicity of the Brucella Cell Wall
Almost all members of the genus Brucella follow this scheme; however, with serious biological, biochemical, and subsequent serological consequences, similarly to other Gram-negative bacteria [10], there are some mutant Brucella species lacking the vast outer lipopolysaccharide layer [11]. These mutants were identified as R (rough)-type Brucellas in contrast with the S (smooth)-type species, based on the visual characterization of bacterial colonies grown on solid media. The virulence of R-type mutants is radically weakened due to the mutations not detailed here [11], making some of them applicable as Brucella vaccines.
According to their low prevalence, their importance from the standpoint of brucellosis serology is generally low. However, their application as vaccines, while providing opportunity for monitoring vaccination, could simultaneously prove to be an obstacle in the serologic distinction between vaccinated and infected populations.
The prevalence of the S-type Brucella species is substantially higher, hence false positivity in Brucella serology is mainly contributed by S-types. Therefore, in the following chapters, the biochemical and antigenic character of smooth Brucella spp. is discussed.
The description of the cell envelope will proceed from the internal parts of a bacterium (cytoplasm) to the extracellular space. Biological roles, structures, and cellular mechanisms involved in the generation of cytoplasmic proteins will not be detailed, only their antigenic characteristics will be discussed below as applicable [12][13][14]. The cytoplasm is surrounded by the inner membrane, built as a double layer (bilayer) from phospholipids and directly covers the cytoplasmic space, and is thus also referred to as the cytoplasmic membrane. There are various membrane proteins wedged into the inner membrane but, similarly to the protein content of the cytoplasm, only their antigenic characteristics will be considered [13]. At the outer side of the inner membrane, reswearchers find the periplasm: a structured, gel-like space with a peptidoglycan membrane distinctly different from the peptidoglycan membrane of Gram-positive bacteria, as it is not multilayered and is thus substantially thinner and more fragile. There are proteins dissolved in the periplasmic fluid, including enzymes (hydrolases, antibiotic-degrading enzymes, etc.), heavy metal neutralizers, carrier proteins, and bacterial toxin subunits. As in the case of cytoplasmic and inner membrane proteins, only their antigenic characteristics will be detailed as relevant to this research paper [15].
The outermost part of the envelope is the cell wall composed of a phospholipid membrane monolayer and an associated lipopolysaccharide (LPS) layer. The outer membrane also anchors the periplasmic peptidoglycan layer by specific proteins: the outer-membrane proteins (OMPs). Some OMPs belong to the porin protein family providing molecular communication across the cell wall with their typical β-barrel (a tube-like structure of antiparallel β-folds) structure [16][17][18]. A detailed presentation of OMPs and their potential in Brucella serology will be provided below. Recent studies have proven that lipopolysaccharide (LPS) is inhomogeneous: a mixture of full-length polysaccharide chains (S LPS) and truncated forms (R LPS) clustered around OMPs [10][19].
The asymmetric composition of the outer membrane can lead to the extremely increased hydrophobicity of the bacterium particle in all cases where the LPS becomes thinner due to mutations, causing the tendency for spontaneous congealing—similarly in the case of rough mutations.
The long (built from dozens of monosaccharides) polysaccharide chains of the LPS form a protective fur-like layer, making it difficult for hydrophobic molecules to penetrate the outer membrane and enter the periplasm. This strong fur coat lures the host immune system during the early phase of the infection and protects the Brucella cells from monocyte phagocytosis later, as referred below.
The LPS consists of three main elements distinct in composition, structure, and function. These elements, in order, starting from the interior of the bacterium cell toward the extracellular space are: (i) the O-specific polysaccharide (OPS), (ii) the core oligosaccharide (COS), and (iii) the so-called lipid A. Figure 1 presents the physical assembly of the main components only, without the detailed biochemical and biophysical processes (enzyme reactions, biochemical pathways, cell trafficking routes, and their elements [20]), resulting in the final composition and structure.
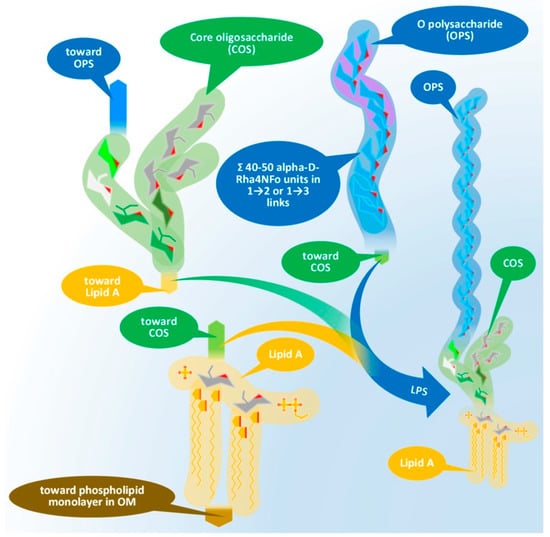
Figure 1. Physical assembly of LPS of Gram-negative bacteria demonstrated by the elements of Brucella LPS. Chemical compositions of the main components will be discussed later. LPS: lipopolysaccharide, OPS: O-specific polysaccharide, COS: core oligosaccharide, OM: outer membrane.
Lipid A, similar to phospholipids, consists of a polar head and a hydrophobic base. It is the key feature enabling the building of the asymmetric membrane bilayer of the outer membrane (OM). It is a conservative structure among Gram-negative bacteria, as detailed in Figure 2 and in the short discussion following the figure.
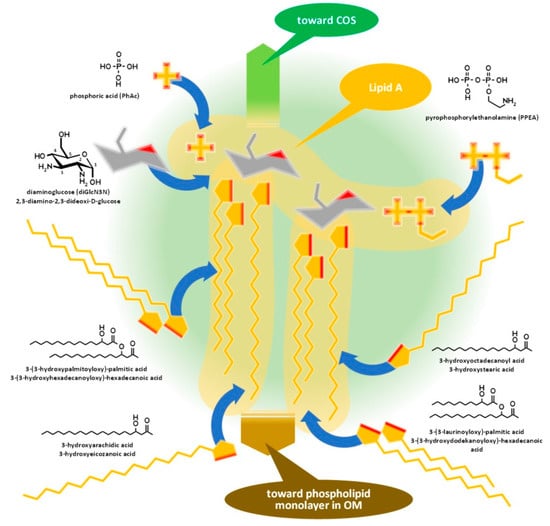
Figure 2.
Assembly of lipid A of Gram-negative bacteria demonstrated by the composition of
Brucella
lipid A. COS: Core oligosaccharide, OM: outer membrane.
Figure 3 and its discussion presents the core oligosaccharide (COS), which, as its name suggests, is a core that links together subunits of LPS with different structures and functions, connecting the lipid A and the O-specific polysaccharide. Molecular diversity of the COS is high among bacterial species, including the strains of species.
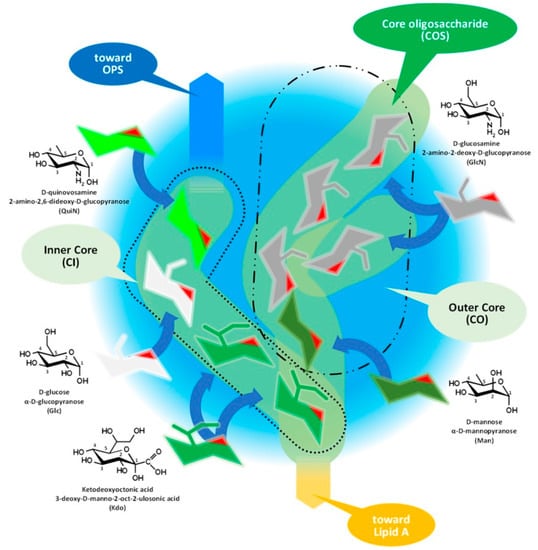
Figure 3.
Assembly of core oligosaccharide of Gram-negative bacteria demonstrated by the composition of
Brucella
COS. OPS: O-specific polysaccharide.
The outmost part of the LPS, the O-specific polysaccharide (OPS), detailed by Figure 4 and Figure 5, is also known as O-specific side chain or O antigen. The OPS is composed of a high number of repeating subunits built of 2–7 sugar components of various compositions. The pattern of subunit repetitions is characteristic of bacterial species, resulting in high antigen variability, which can serve as the basis for the serological grouping of Gram-negative bacteria. The number of sugar moieties (which is dramatically reduced in rough mutants) in the O-specific polysaccharide chain determines the morphology of the bacterial colony, i.e., smooth or rough colony types with and without lengthy OPS chains, respectively. LPS which is lacking OPS due to a low degree of polymerization could be referred to as lipooligosaccharide—LOS.
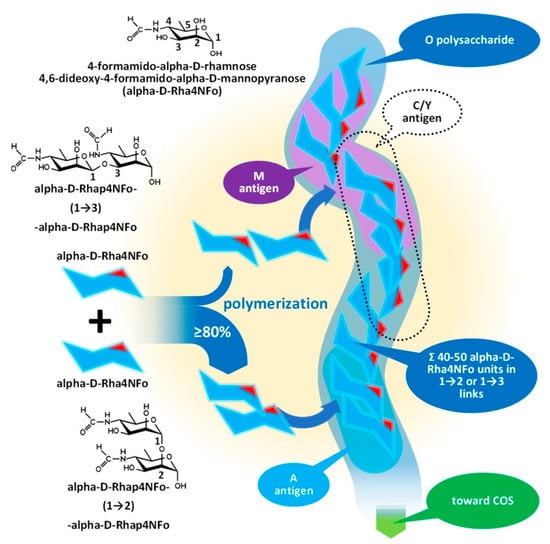
Figure 4.
Assembly of the O-polysaccharide of Gram-negative bacteria demonstrated by the composition of
Brucella
OPS. COS: Core oligosaccharide.
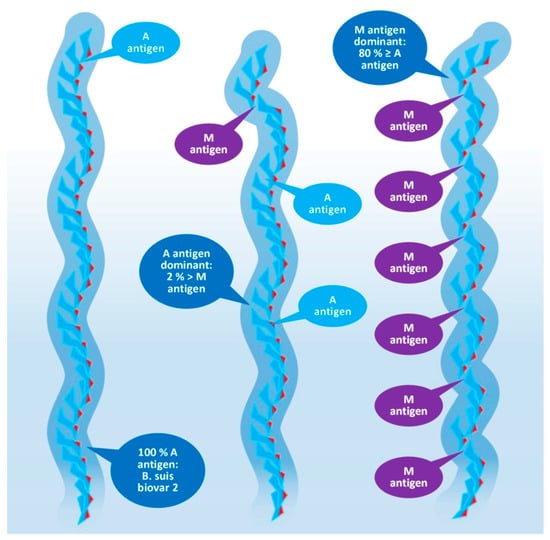
Figure 5.
Composition of the O-polysaccharide of
Brucella
species with a different antigenic character.
Lipid A is usually built from a bisphosphorylated disaccharide with a rather conservative structure (the hydrophilic head), acylated with various fatty acid chains with variable lengths and branching, providing the hydrophobic base that noncovalently sticks to the hydrophobic side of the phospholipid monolayer in the OM.
Although it seems that lipid A structures, being the most deeply buried part of the LPS and embedded into the OM, have minor importance from a serologic point of view, as main determinants of bacterial endotoxicity—especially the low endotoxicity of brucellae, which amplify the stealthing ability of the bacteria [9][21][22]—some discussion of their antigenicity is required.
Lipid A of brucellae is the most hydrophobic membrane anchor among Gram-negative bacteria. The typical length of fatty acid chains in Brucella abortus is 16–18 with some very long (28–30) substituents [23][24][25] in contrast with the typical C12–C14 chains of enterobacteria [21][26]. The six long fatty acid substituents make it difficult to release the endotoxin for Brucella, while Y. enterocolitica lipid A, with only four and substantially shorter fatty acid chains, is a good source of free endotoxins.
The intracellular lifestyle that S-type brucellae enjoy in the endoplasmic reticulum [22] can be supported both by the firm anchorage of the LPS through lipid A—a useful feature to prevent dissolution in a lysosome as it generally happens with R-type bacteria —and by the attenuated host immune response [7][8][22]. Although diverse explanations have been published on the exact role of the LPS in such attenuations, data demonstrating the existence of S LPS-MHC II complexes [22][27][28][29] suggest a contribution by the extremely hydrophobic lipid A.
The core oligosaccharide has branched chains with two structurally different subunits, as Figure 3 demonstrates on the structure of Brucella COS, which are: (i) the inner core (CI) and (ii) the outer core (CO). ResWearchers should mention that this classification of the subunits could be obsolete in the light of recent studies. The composition of the CI consists of rare sugar moieties, such as the characteristic ketodeoxyoctonic acid (Kdo) and heptose sugars or—such as in the case of Brucella—D-quinovosamine (QuiN) [30].
The terminal Kdo attaches the COS to the lipid A through a ketosidic bond, is sensitive to weak acids, and provides a link to the outer core as well. In the CO, the number of hexose monomers is variable with six sugar moieties as a maximum. Depending on the number of monomers, the CO could contain further small branches—in the case of Brucella, through the side chain of a D-glucosamine. A terminal moiety of a CI (such as in the case of Y. enterocolitica serotype O:3 and Brucella spp.) or a CO (E. coli and Salmonella spp.) branch attaches the COS to the OPS [21][30][31][32][33][34].
Investigations of the so-called deep rough mutants, such as the D31m4 of E. coli [35], determined the minimum length of the full COS in viable Gram-negative bacteria: two heptoses, usually Kdo monomers, should be attached to lipid A [35]. Rare exceptions exist, as in the case of Helicobacter pylori, with only one Kdo linked to lipid A.
The diverse composition and the branched structure of COS could serve as a good basis for antigenic differentiation; however, utilizing this diversity is difficult for serologic applications in the case of S-type bacteria as the majority of COS remain cryptic under the OPS layer. The mentioned diversity of the OPS chain length and the inhomogeneous cell surface location of the different OPS molecules, that is the clustering of R LPS molecules around OMPs [10][19], increases the theoretical possibility of an immune response against COS motifs on the intact cells and serologic detection of antibodies involved in such a response.
The O-specific polysaccharide as the outer layer of the cell wall is the main antigen determinant of the LPS and provides a solid, yet relatively unreliable, basis for the serologic differentiation of Gram-negative bacteria species and strains [36]. The molecular and topological diversity of the OPS is theoretically high: more than five dozen kinds of sugar moieties were identified as constituents of the polymer in various numbers, proportions, and clusters, built into linear or branched chains, and linked to even non-sugary substituents [27][37].
More than 180 different O-serotypes of E. coli have been identified before the high throughput genotyping era until 2005 [37] due to this variability, and, paradoxically, the high molecular diversity results in a lower antigenic diversity in several cases [37]. E. coli O35 and Salmonella enterica O62 or E. coli O98 and Yersinia enterocolitica O11,24 or E. coli O8 and Klebsiella pneumoniae O5 and our current subject, Brucella spp., E. coli O157:H7 and Y. enterocolitica O9 have identical or nearly identical antigens; in the last case, identical enough to present false positive serologic results.
The O-specific polysaccharide of the Y. enterocolitica O9 and the brucellae (see Figure 4) is characteristically a homopolymer of 4,6-dideoxy-4-formamido-alpha- D-mannopyranose (alpha-D-Rha4NFo) sugar moieties, which are linked to each other through α(1→2) and/or α(1→3) bonds in different proportions. As is shown in Figure 5, the prevalence of α(1→2) and α(1→3) bonds are different in the strains of Brucella spp. with an average proportion of the α(1→3) links between 0 and 20 percent. The first saccharide monomer of the OPS always has a reducing end and is linked to the next sugar through an α(1→2) bond. The serologic importance of the terminus of the OPS is high [38].
Principally, the serologic differentiation of Gram-negative bacterial strains is based on antibody recognition of repeating oligomeric saccharide motifs in the OPS rather than individual sugar moieties. As in the case of brucellae, whose serologic division was among the earlier classification attempts targeting bacterium species (carried out in 1932 by Wilson [39]), it may have been the very first one, preceding the serotyping of E. coli in 1944, or Salmonella spp. in 1935, during the extensive and pioneering efforts of Kaufman [40][41][42][43]. The early serotypes, M after Brucella melitensis and A after Brucella abortus, provided the basis for the later developed Brucella M and A antigen grouping and led to the recent stage of knowledge summarized in Figure 4 and Figure 5 [38][44][45].
Brucella OPS could carry the antigens A, M, C/Y, and C. According to recent knowledge [44], the A antigen represents an alpha-D-Rha4NFo moiety, which is linked between two alpha-D-Rha4NFo saccharides through α(1→2) bonds; or, suggesting a more simple determination: A antigen is two alpha-D-Rha4NFo moieties linked together by an α(1→2) bond (see Figure 4). The M antigen represents a cluster of four alpha-D-Rha4NFo moieties with a central link of an α(1→3) bond and the two saccharides on the termini of the cluster is linked by α(1→2) bonds; or, with a more simple determination: two A antigens linked together with an α(1→3) bond. As it is shown in Figure 5, at least one bacterium exists in the genus Brucella with uniform antigenicity: B. suis biovar 2 contains exclusively A antigens [46]. With similar rare exceptions, near the cap of the brucellae OPS, usually an α(1→3) bond can be found [38].
This molecular-based antigen division provides more sensitive typing since the determination of M and A dominant strains based on A/M ratios could identify strains between two extremis: the 100% A dominant B. suis biovar 2 and the B. melitensis 16M containing 21% of M [47].
The very existence of the C/Y and C antigens is still debated. Theoretically, they are characterized as overlapped A and M antigens with different proportions, that is, C/Y (as a common antigen of some members of the genus Brucella and Y. enterocolitica O:9) has more A than M and in C antigen A = M [48]—if rwesearchers retain the fish and bait metaphor, they are baits for fish with the largest mouths—consequently with a lower target sensitivity.
Due to its chemical nature, which is abundantly composed of saccharides, and the rather narrow repertoire of sugar moieties occurring across bacterial families, from a serological point of view, the outermost polysaccharide component of the protective LPS layer seems to be overly uniform in the case of smooth Gram-negative bacteria; consequently, the application of isolated antigens with S LPS origin in serological tests almost automatically provides false positives. The baits used are evidently far from perfection.
References
- Herron, J.B.T.; Dunbar, J.A.T. The British Army’s contribution to tropical medicine. Clin. Med. 2018, 18, 380–383.
- Wright, A.; Smith, F. On the application of the serum test to the differential diagnosis of typhoid and Malta fever: And on the further application of the method of serum diagnosis to the elucidation of certain problems in connexion with the duration of immunity and the geographical distribution of disease. Lancet 1897, 1, 656–659.
- Wright, A.E.; Semple, D. On the employment of dead bacteria in the serum diagnosis of typhoid and Malta fever, and on an easy method of extemporising a blowpipe flame for making capillary sero-sedimentation tubes. Brit. Med. J. 1897, 1, 1214–1215.
- Scholz, H.C.; Nöckler, K.; Göllner, C.; Bahn, P.; Vergnaud, G.; Tomaso, H.; Al Dahouk, S.; Kämpfer, P.; Cloeckaert, A.; Maquart, M.; et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 2010, 60, 801–808.
- Brucellosis in Sheep and Goats-European Commission, Health & Consumer Protection Directorate-General. SANCO.C.2/AH/R23/2001. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scah_out59_en.pdf (accessed on 12 July 2001).
- Celli, J. The intracellular life cycle of Brucella spp. Microbiol. Spectr. 2019, 7, 101–111.
- Martirosyan, A.; Moreno, E.; Gorvel, J.P. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 2011, 240, 211–234.
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzmán-Verri, C.; Chacón-Díaz, C.; Rucavado, A.; Moriyón, I.; Moreno, E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2007, 2, e631.
- Leong, D.; Diaz, R.; Milner, K.; Rudbach, J.; Wilson, J.B. Some structural and biological properties of Brucella endotoxin. Infect. Immun. 1970, 1, 174–182.
- Gammoudi, I.; Mathelie-Guinlet, M.; Morote, F.; Beven, L.; Moynet, D.; Grauby-Heywang, C.; Cohen-Bouhacina, T. Morphological and nanostructural surface changes in Escherichia coli over time, monitored by atomic force microscopy. Colloids Surf. B Biointerfaces 2016, 141, 355–364.
- Huber, M.; Kalis, C.; Keck, S.; Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Beutler, B.; et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur. J. Immunol. 2006, 36, 701–711.
- Han, X.; Tong, Y.; Tian, M.; Sun, X.; Wang, S.; Ding, C.; Yu, S. Characterization of the immunogenicity and pathogenicity of malate dehydrogenase in Brucella abortus. World J. Microbiol. Biotechnol. 2014, 30, 2063–2070.
- Faria, A.R.; Dorneles, E.M.S.; Pires, S.D.F.; de Andrade, H.M.; Lage, A.P. Immunoproteomics of Brucella abortus reveals potential of recombinant antigens for discriminating vaccinated from naturally infected cattle. Microb. Pathog. 2020, 147, 104345.
- Hop, H.T.; Arayan, L.T.; Simborio, H.L.; Reyes, A.W.; Min, W.; Lee, H.J.; Lee, J.J.; Chang, H.H.; Kim, S. An evaluation of ELISA using recombinant Brucella abortus bacterioferritin (Bfr) for bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 2016, 45, 16–19.
- Nagalingam, M.; Basheer, T.J.; Balamurugan, V.; Shome, R.; Kumari, S.S.; Reddy, G.; Shome, B.R.; Rahman, H.; Roy, P.; Kingston, J.J.; et al. Comparative evaluation of the immunodominant proteins of Brucella abortus for the diagnosis of cattle brucellosis. Vet. World 2021, 14, 803–812.
- Bulashev, A.; Akibekov, O.; Syzdykova, A.; Suranshiyev, Z.; Ingirbay, B. Use of recombinant Brucella outer membrane proteins 19, 25, and 31 for serodiagnosis of bovine brucellosis. Vet. World 2020, 13, 1439–1447.
- Vatankhah, M.; Beheshti, N.; Mirkalantari, S.; Khoramabadi, N.; Aghababa, H.; Mahdavi, M. Recombinant Omp2b antigen-based ELISA is an efficient tool for specific serodiagnosis of animal brucellosis. Braz. J. Microbiol. 2019, 50, 979–984.
- Koyuncu, I.; Kocyigit, A.; Ozer, A.; Selek, S.; Kirmit, A.; Karsen, H. Diagnostic potential of Brucella melitensis Rev1 native Omp28 precursor in human brucellosis. Central Eur. J. Immunol. 2018, 43, 81–89.
- Vassen, V.; Valotteau, C.; Feuillie, C.; Formosa-Dague, C.; Dufrêne, Y.F.; De Bolle, X. Localized incorporation of outer membrane components in the pathogen Brucella abortus. EMBO J. 2019, 38, e100323.
- Bohl, H.O.; Aihara, H. Current progress in the structural and biochemical characterization of proteins involved in the assembly of lipopolysaccharide. Int. J. Microbiol. 2018, 2018, 5319146.
- Trent, M.S.; Stead, C.M.; Tran, A.X.; Hankins, J.V. Diversity of endotoxin and its impact on pathogenesis. J. Endotoxin Res. 2006, 12, 205–223.
- Ahmed, W.; Zheng, K.; Liu, Z.F. Establishment of chronic infection: Brucella’s stealth strategy. Front. Cell. Infect. Microbiol. 2016, 6, 30.
- Ferguson, G.P.; Datta, A.; Baumgartner, J.; Roop, R.M.; Carlson, R.W.; Walker, G.C. Similarity to peroxisomal-membrane protein family reveals that Sinorhizobium and Brucella BacA affect lipid-A fatty acids. Proc. Natl. Acad. Sci. USA 2004, 101, 5012–5017.
- Moreno, E.; Stackebrandt, E.; Dorsch, M.; Wolters, J.; Busch, M.; Mayer, H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J. Bacteriol. 1990, 172, 3569–3576.
- Cardoso, P.G.; Macedo, G.C.; Azevedo, V.; Oliveira, S.C. Brucella spp. noncanonical LPS: Structure, biosynthesis, and interaction with host immune system. Microb. Cell. Factories 2006, 5, 13.
- Tsolis, R.M.; Young, G.M.; Solnick, J.V.; Bäumler, A.J. From bench to bedside: Stealth of enteroinvasive pathogens. Nat. Rev. Microbiol. 2008, 6, 883–892.
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identification. OCL 2020, 27, 31.
- Lapaque, N.; Moriyon, I.; Moreno, E.; Gorvel, J.P. Brucella lipopolysaccharide acts as a virulence factor. Curr. Opin. Microbiol. 2005, 8, 60–66.
- Forestier, C.; Moreno, E.; Méresse, S.; Phalipon, A.; Olive, D.; Sansonetti, P.; Gorvel, J.-P. Interaction of Brucella abortus lipopolysaccharide with major histocompatibility complex class II molecules in B lymphocytes. Infect. Immun. 1999, 67, 4048–4054.
- Wilkinson, S.G. Bacterial lipopolysaccharides—Themes and variations. Prog. Lipid Res. 1996, 35, 283–343.
- Frirdich, E.; Whitfield, C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J. Endotoxin Res. 2005, 11, 133–144.
- Gil-Ramírez, Y.; Conde-Álvarez, R.; Palacios-Chaves, L.; Zúñiga-Ripa, A.; Grilló, M.J.; Arce-Gorvel, V.; Hanniffy, S.; Moriyón, I.; Iriarte, M. The identification of wadB, a new glycosyltransferase gene, confirms the branched structure and the role in virulence of the lipopolysaccharide core of Brucella abortus. Microb. Pathog. 2014, 73, 53–59.
- Fontana, C.; Conde-Álvarez, R.; Ståhle, J.; Holst, O.; Iriarte, M.; Zhao, Y.; Arce-Gorvel, V.; Hanniffy, S.; Gorvel, J.-P.; Moriyón, I.; et al. Structural studies of lipopolysaccharide-defective mutants from Brucella melitensis identify a core oligosaccharide critical in virulence. J. Biol. Chem. 2016, 291, 7727–7741.
- Fontana, C.; Conde-Álvarez, R.; Ståhle, J.; Holst, O.; Iriarte, M.; Zhao, Y.; Arce-Gorvel, V.; Hanniffy, S.; Gorvel, J.-P.; Moriyón, I.; et al. A new Brucella lipopolysaccharide core glycosyltransferase identified by genomic search and phenotypic characterization. Front. Microbiol. 2018, 9, 2293.
- Qureshi, N.; Takayama, K.; Mascagni, P.; Honovich, J.; Wong, R.; Cotter, R.J. Complete structural determination of lipopolysaccharide obtained from deep rough mutant of Escherichia coli. Purification by high performance liquid chromatography and direct analysis by plasma desorption mass spectrometry. J. Biol. Chem. 1988, 263, 11971–11976.
- DebRoy, C.; Fratamico, P.M.; Yan, X.; Baranzoni, G.; Liu, Y.; Needleman, D.S.; Tebbs, R.; O’Connell, C.D.; Allred, A.; Swimley, M.; et al. Comparison of O-antigen gene clusters of all O-serogroups of Escherichia coli and proposal for adopting a new nomenclature for O-typing. PLoS ONE 2016, 11, e0147434.
- Stenutz, R.; Weintraub, A.; Widmalm, G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 2006, 30, 382–403.
- McGiven, J.; Howells, L.; Duncombe, L.; Stack, J.; Ganesh, N.V.; Guiard, J.; Bundle, D.R. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping m epitope elements of Brucella O-polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210.
- Wilson, G.S.; Miles, A.A. The serological differentiation of smooth strains of the Brucella group. Br. J. Exp. Pathol. 1932, 13, 1–13.
- Kaufmann, F. Zur serologie der Coli gruppe. Acta Pathol. Microbiol. Scand. 2009, 21, 20–45.
- Kauffmann, F. Ueber einen neuen serologischen formenwechsel der Typhusbacillen. Z. Hyg. Infektionskr. 1935, 116, 617–651.
- Kauffmann, F. Untersuchungen uber die korperantigene in der Salmonella-gruppe. Z. Hyg. Infektionskr. 1936, 117, 778–791.
- Kauffmann, F. On the serology of the Klebsiella group. Acta Pathol. Microbiol. Scand. 1949, 26, 381–406.
- Bundle, D.R.; McGiven, J. Brucellosis: Improved diagnostics and vaccine insights from synthetic glycans. Acc. Chem. Res. 2017, 50, 2958–2967.
- Kubler-Kielb, J.; Vinogradov, E. Reinvestigation of the structure of Brucella O-antigens. Carbohydr. Res. 2013, 378, 144–147.
- Zaccheus, M.V.; Ali, T.; Cloeckaert, A.; Zygmunt, M.S.; Weintraub, A.; Iriarte, M.; Moriyón, I.; Widmalm, G. The epitopic and structural characterization of Brucella suis biovar 2 O-polysaccharide demonstrates the existence of a new M-negative C-negative smooth Brucella serovar. PLoS ONE 2013, 8, e53941.
- Ganesh, N.V.; Sadowska, J.M.; Sarkar, S.; Howells, L.; McGiven, J.; Bundle, D.R. Molecular recognition of Brucella A and M antigens dissected by synthetic oligosaccharide glycoconjugates leads to a disaccharide diagnostic for brucellosis. J. Am. Chem. Soc. 2014, 136, 16260–16269.
- Zygmunt, M.S.; Bundle, D.R.; Ganesh, N.V.; Guiard, J.; Cloeckaert, A. Monoclonal antibody-defined specific C epitope of Brucella O-polysaccharide revisited. Clin. Vaccine Immunol. 2015, 22, 979–982.
More
