Cannabis sativa is a multipurpose plant that has been used in medicine for centuries. Recently, cConsiderable research has focused on the bioactive compounds of this plant, particularly cannabinoids and terpenes. Among other properties, these compounds exhibit antitumor effects in several cancer types, including colorectal cancer (CRC). Cannabinoids show positive effects in the treatment of CRC by inducing apoptosis, proliferation, metastasis, inflammation, angiogenesis, oxidative stress, and autophagy. Terpenes, such as β-caryophyllene, limonene, and myrcene, have also been reported to have potential antitumor effects on CRC through the induction of apoptosis, the inhibition of cell proliferation, and angiogenesis. In addition, synergy effects between cannabinoids and terpenes are believed to be important factors in the treatment of CRC.
- Cannabis sativa
- colorectal cancer
- cannabinoids
- terpenes
- apoptosis
- proliferation
- metastasis
- inflammation
- angiogenesis
- oxidative stress
1. Introduction
2. Bioactive Compounds of Cannabis sativa
Among the multiple bioactive compounds found in C. sativa, the main ones are cannabinoids, terpenoids, flavonoids, stilbenoids, and alkaloids [19,20][11][12]. When consumed, these substances can induce a variety of beneficial health effects and are thought to contribute to the plant’s therapeutic qualities. As for natural products in general, the phytochemical content of C. sativa varies, depending on distinct factors, including genetics, growing conditions, stage of growth, harvest time, processing, and storage, among others [21][13]. C. ruderalis and C. indica contain a smaller amount of cannabidiol (CBD) than C. sativa. In contrast, C. indica has the largest THC content compared to C. sativa, and C. ruderalis has the lowest [2].2.1. Cannabinoids
Cannabinoids are a type of terpenophenolic compounds with a C21 backbone [22][14]. The diversity of chemical structures of phytocannabinoids is mainly due to the differences between the isoprenyl groups, the side chain and the resorcinyl core [23][15]. Cannabinoids, according to their chemical structure, can be classified into 11 different classes: cannabigerol (CBG), cannabidiol (CBD), cannabichromene (CBC), Δ9-THC, Δ8-THC, cannabicyclol (CBL), cannabinol (CBN), cannabinodiol (CBND), cannabielsoin (CBE), cannabitriol (CBT), and miscellaneous-type cannabinoids [22,24][14][16]. Most cannabinoids originate from cannabigerolic acid (CBGA), as revealed in Figure 1. Its biosynthesis occurs with the production of CBGA from the alkylation of olivetolic acid with geranyl pyrophosphate (GPP). The primary cannabinoids, cannabichromenic acid (CBCA), cannabidiolic acid (CBDA), and ∆9-tetrahydrocannabinolic acid (∆9-THCA), are synthesized in acid form by the enzymes CBCA synthase, CBDA synthase, and THCA synthase. Decarboxylation then converts the acidic forms of cannabinoids into neutral forms (THC, CBD, and CBC), which are recognized for having higher pharmacological potential [2,25,26,27][2][17][18][19]. This conversion typically occurs naturally as the plant matures and dries, or because of heat exposure during processing or cooking [28][20].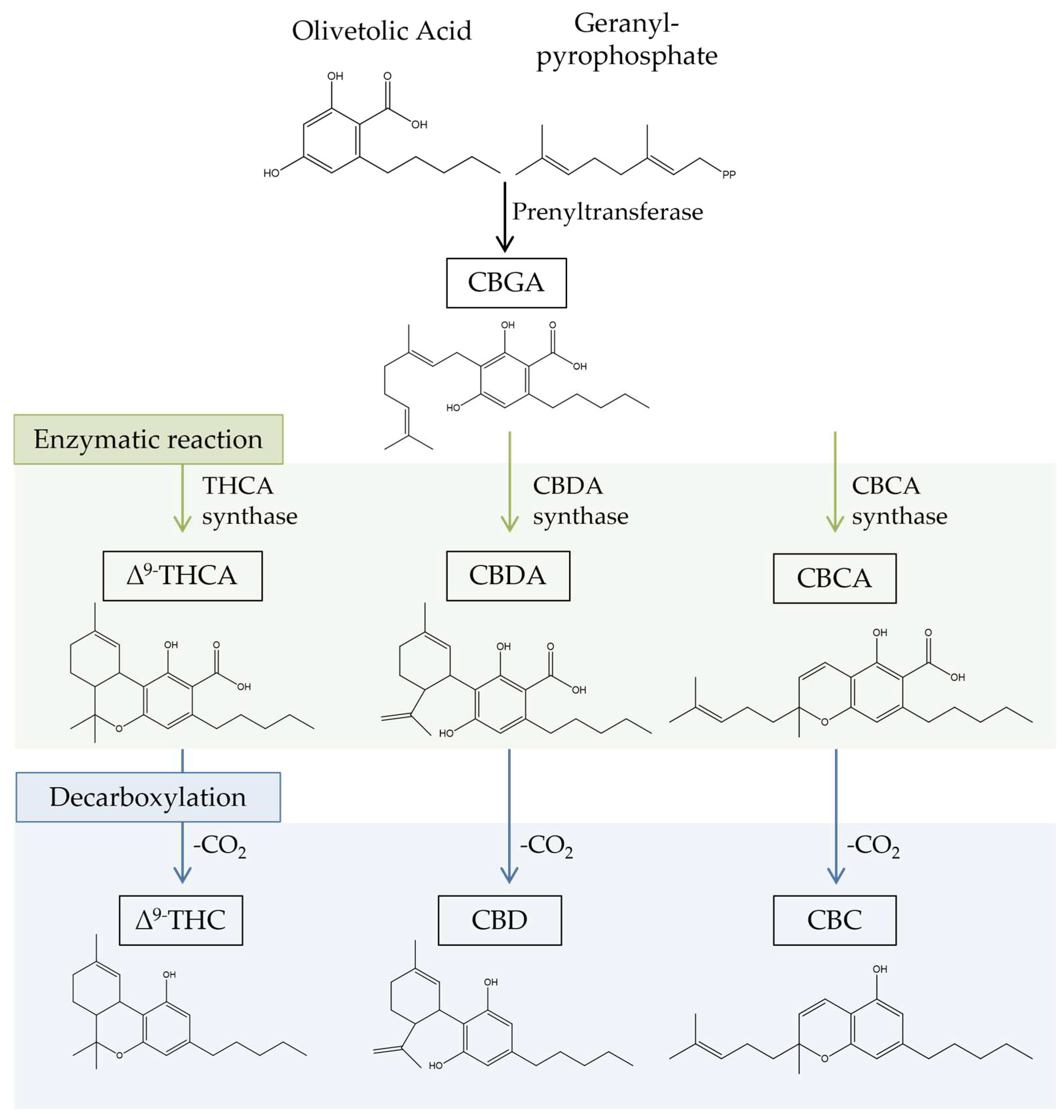
2.2. Terpenes
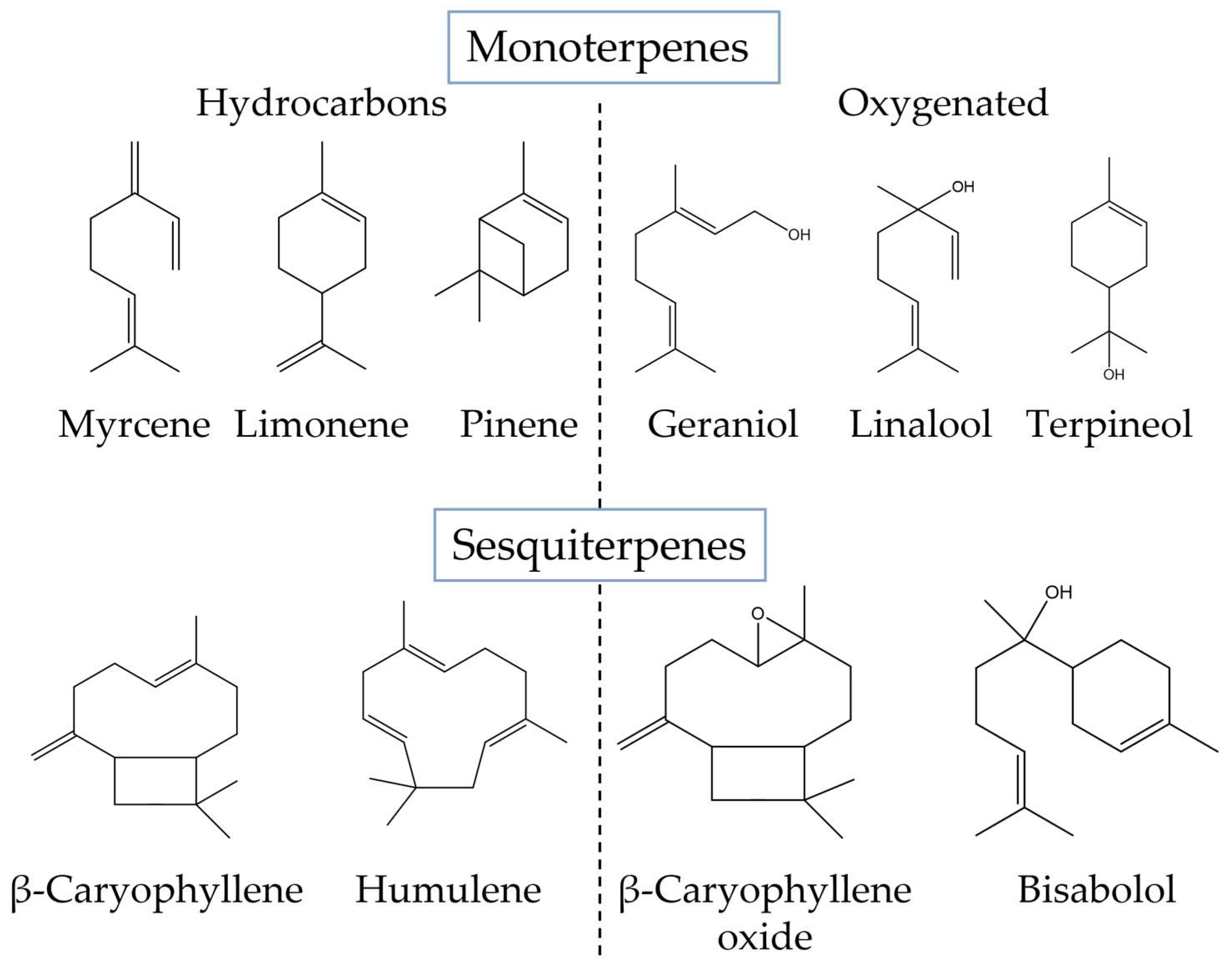
Terpenes, characterized by multiple five-carbon isoprene units linked together to form a chain of hydrocarbons, comprise the second-largest class of plant constituents (120 identified so far). These secondary metabolites are synthesized via the isoprenoid biosynthesis metabolic pathway (Figure 2). Isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP), which are generated through the mevalonic acid (MEV) or methylerythritol phosphate (MEP) pathway, serve as the precursor molecules for this pathway. Afterward, a series of enzyme-catalyzed processes transform the IPP and DMAPP into several terpene precursors, including GPP, farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP). GPP is responsible for the production of terpene molecules, which are then used in the production of cannabinoids. Different terpene synthases can further modify these precursors to produce a variety of monoterpenes, sesquiterpenes, and diterpenes [38,39][21][22].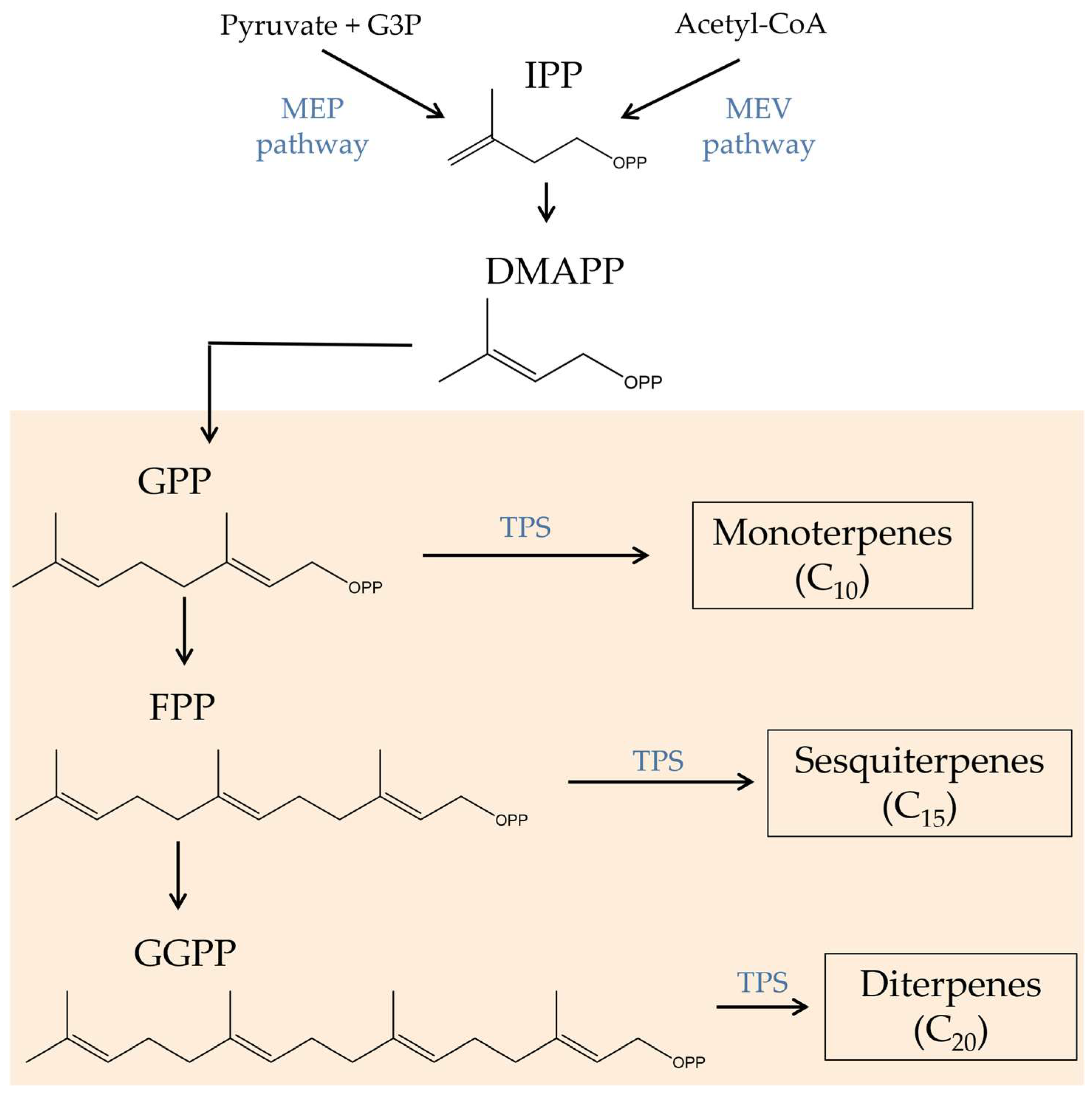
3. Bioactive Compounds in the Prevention and Treatment of Colorectal Cancer
3. Bioactive Compounds in the Prevention and Treatment of Colorectal Cancer
3.1. Effects of Cannabinoids in CRC-Associated Mechanisms
3.1. Effects of Cannabinoids in CRC-Associated Mechanisms
Over hir history, there has been increasing evidence of cannabis’s beneficial effects on CRC. Several in vitro and in vivo experiments showed that natural phytocannabinoids from C. sativa can interact with some of the mechanisms inherent in cancer. Among them, apoptosis, autophagy, inflammation, migration, invasion, and metastasis are the target mechanisms that have demonstrated better outcomes in CRC carcinogenesis with cannabinoids (Table 3). As mentioned earlier, cannabinoids can exert anticancer effects in part due to their interaction with the ECS. This is a complex cell signaling system present in all mammals, which is involved in regulating various physiological and cognitive processes, such as appetite, pain, mood, and memory. It is composed of three main components: endocannabinoids, receptors, and metabolic enzymes. Endocannabinoids are lipid-based neurotransmitters produced by the body, such as anandamide and 2-arachidonoylglycerol (2-AG), which bind to cannabinoid receptors (CB1 and CB2) found on the surface of cells, triggering a cellular response. Cannabinoids then can act on the ECS by mimicking endocannabinoids and binding to CB1 and CB2 receptors. Other receptors interact with endocannabinoids and modulate the ECS, including G-protein-coupled receptor 55 (GPR55), transient receptor potential cation channel subfamily V member 1 (TRPV1), TRPV2, transient receptor potential cation channel subfamily M member 8 (TRPM8), and peroxisome proliferator-activated receptors (PPARs) (Figure 4) [49][32]. The role of ECS in CRC has been reviewed elsewhere [17][33]. However, it is important to note that CRC cells and tissues express both CB1 and CB2 receptors, and TRPM8, TRPA1, TRPV1, and TRPV2 receptors, to which cannabinoids can bind to exert biological effects on CRC [12,17][27][33].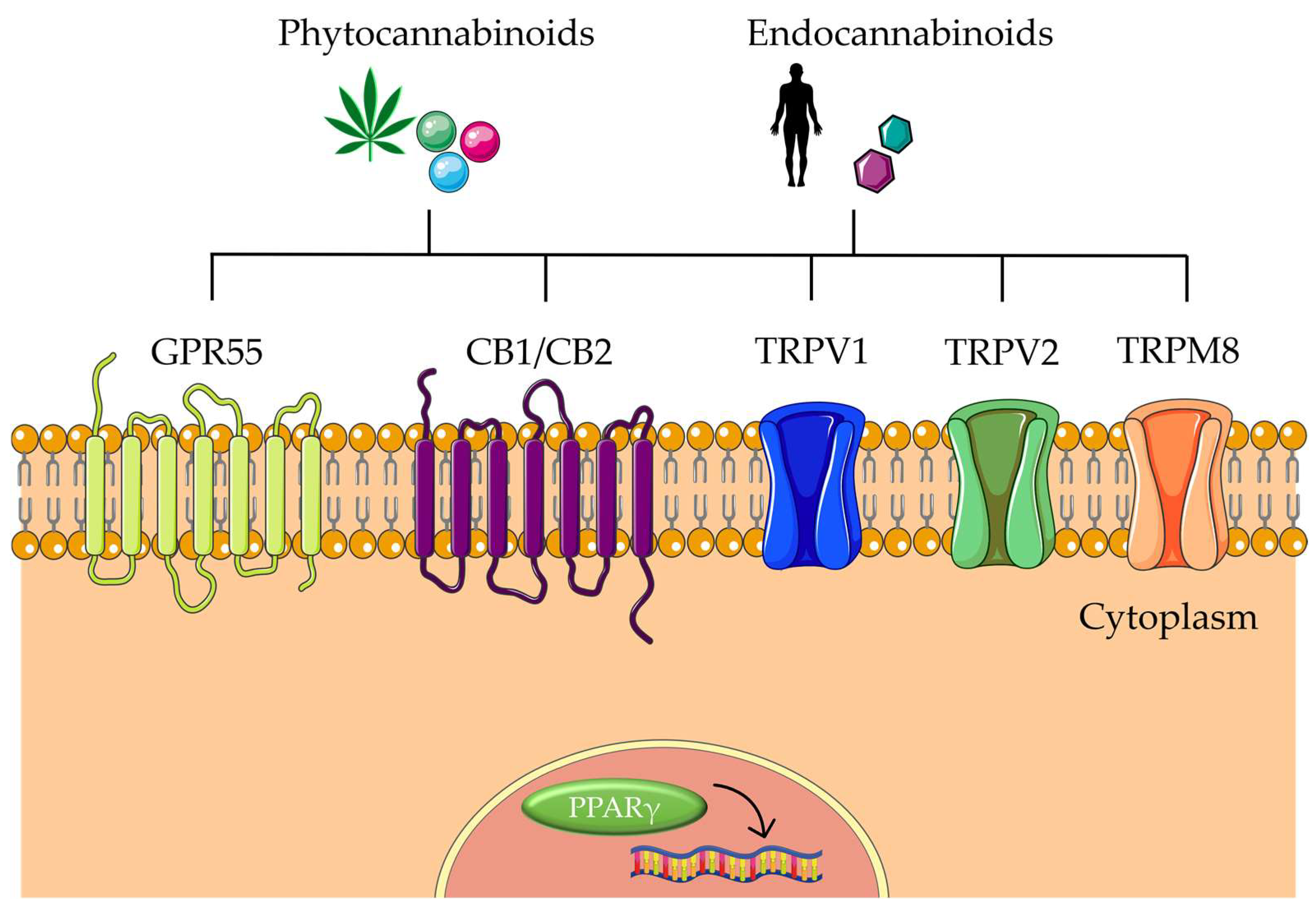
3.1.1. CBD
3.1.2. Δ9-THC
THC has also been reported to have good outcomes in inhibiting cell viability on CRC cells, namely, SW480, HCT-15, HT-29, and HCA7 [52,64][36][46]. In HT-29 cells, THC suppressed cell viability more than CB83 (a synthetic cannabinoid) after 24 h of treatment at 30 µM [52][36]. Additionally, Shor and coworkers reported that Δ9-THC and Δ8-THC were more toxic to polyp-derived cells than other cannabinoids, namely, CBD, CBC, CBDV, THCA, CBG, and other minor cannabinoids [65][47]. Furthermore, THC at 30 µM was shown to trigger significant inhibition of proliferation at 24 h (70.9 ± 5.59, p < 0.01) in HT-29 CRC cells, compared to untreated cells [52][36]. The induction of apoptosis by THC through the activation of the CB1 receptor in CRC cells was first reported in 2007 by Greenhoug et al. [64][46]. The authors suggested that apoptosis was induced by the activation of the B-cell lymphoma-2 (Bcl-2) family member BAD protein dependent on the CB1-mediated Ras-mitogen-activated protein kinase and PI3K/Akt pathway inhibition. In reality, the inhibition of ERK and Akt activity by THC was complemented by activation of the proapoptotic BAD. Additionally, THC increased the simultaneous cleavage of the caspase-3 substrate PARP. Optimizing the delivery method can improve the efficacy of THC treatment for CRC owing to its lipophilicity, tar-like viscosity, and instability. In fact, De la Ossa et al. suggested an alternative method of THC delivery. The oil-in-water emulsion solvent evaporation method used by the authors to encapsulate THC into biodegradable microspheres revealed its ability to prevent cancer cell proliferation in CACO-2 cells [66][48].3.1.3. CBG
CBG, a non-psychoactive cannabinoid, has gained a lot of interest due to its ability to be a partial agonist of CB1 and CB2 receptors [69][49]. In addition to the main receptors of the endocannabinoid system, CBG also acts as an antagonist at TRPM8, TRPV4, and 5HT1A and an agonist at TRPA1, TRPV1, and TRPV2 receptors [70][50]. In CRC, CBG treatment had positive results in cell survival, apoptosis, oxidative stress, and in vivo tumor growth. As for cell survival, CBG was reported to induce a significant decrease in the viability of CACO-2 cells at 30 µM after 3 h of incubation (p < 0.001). However, after 48 h of incubation, CBG effects on cell viability were significantly decreased even for lower concentrations (3, 10, and 30 µM). In HCT116 cells, a similar trend was observed, and this effect was repressed in cells where TRPM8 was suppressed [12][27]. In HCT116 cells, purified CBG exhibited antiproliferative potential at concentrations 2.5, 5, and 10 µM [42][26].3.1.4. Minor Cannabinoids
Despite being less studied, minor cannabinoids such as CBDV, CBL, CBGV, CBCA, THCV, and CBGA revealed positive effects in the cell survival and proliferation of CRC cells. Ben-Ami Shor et al.’s research [65][47] indicated that the combinations of CBCA (14.5 or 29 μM), CBDV (23.5 or 47 μM), THCV (20 or 40 μM), and CBGA (25.6 or 51.2 μM) triggered a decrease in the cell viability of cells derived from human polyps. The combination of CBCA and CBDV also had synergistic effects, as well as the combination of THCV and CBGA. In HCT116 cells, data revealed cell viability proportions of 93, 87, and 51% with CBDV; 69, 44, and 45% with CBL; and 93, 75, and 71% for CBGV treatments at 2.5, 5, and 10 μM, suggesting that these cannabinoids exerted antiproliferative effects against CRC cells [50][34]. Despite the antiproliferative properties demonstrated for the cannabinoids CBCA, CBDV, THCV, and CBGA, the concentrations were relatively high compared to those of CBD, THC, and CBG, suggesting that their effects are not very strong.3.2. Effects of Terpenes on CRC
Terpenes have been screened for their potential to prevent CRC when used alone or, in the case of the C. sativa plant, to enhance the plant’s medicinal properties. These compounds act by inhibiting the growth and proliferation of cancer cells, and by inducing apoptosis. In addition, there is evidence that some terpenoids may have similar effects to cannabinoids, such as THC, in terms of their ability to interact with the endocannabinoid system. This interaction can cause changes in the activity of certain receptors and enzymes, which in turn affect a wide range of physiological processes. Indeed, β-caryophyllene was shown to interact with the CB2 receptor, acting as its full agonist, resulting in a reduction in pain and inflammation in male rats and mice [71][51]. Inflammatory bowel diseases are closely associated with an increased risk of CRC [75][52]. In this sense, Bento et al. [76][53] emphasized that the preventive treatment of DSS-induced colitis CD1 mice with BCP (50 mg/kg) resulted in a significant decrease in TNF-α, IL-1β, keratinocyte-derived chemokine, and interferon-γ protein levels (p < 0.05) in mice colon segments. These proteins are mediators involved in cellular migration and adhesion. The authors also revealed that the decrease in the inflammatory mediators was associated with the factor nuclear kappa B (NFκB) signaling pathway, as BCP decreased the activation of the p65 NFκB subunit.3.3. Effects of Cannabis sativa Extracts on CRC
Interactions between terpenes/terpenoids and cannabinoids may intensify their pharmacological effects. In reality, there is growing evidence that these substances interact more favorably when used in combination than when used alone. This is known as the “entourage effect” in modern parlance, an effect attributed to cannabis’s medical properties [43][54]. Janatová et al. [42][26] suggested that the compounds responsible for the cytotoxicity of C. sativa ethanolic (EtOH) extracts in CACO-2 and HT29 cell lines were THC and CBD. However, in the same work, a specific genotype containing high concentrations of myrcene, β-elemene, β-selinene, and α-bisabolol oxide positively affected the selectivity of cytotoxic activity, due to synergistic effects. In another study conducted by Nallathambi and coworkers, the authors reported an increment in cytotoxic effects of a C. sativa EtOH extract in CRC cells (HCT 116, HT-29, and CACO-2) when this was combined with a THCA-rich fraction [45][28]. In addition to the EtOH extract, fractions obtained from it were also evaluated. The combination of a CBGA-rich fraction and a THCA-rich fraction resulted in a noticeable increase in apoptosis. The CBGA-rich fraction included other compounds such as CBN (3.67%), CBCA (3.53%), terpenes (0.72%), diterpenes (0.33%), and short free fatty acids (0.37%), which may have played a role in the outcome.4. Conclusions
Data suggest that cannabinoids exert advantages in the treatment of CRC, mostly by inducing apoptosis, although some evidence also points out that they may target other key therapeutic events, such as proliferation, metastasis, inflammation, angiogenesis, oxidative stress, and autophagy. The currently available data on this subject refer mostly to the C. sativa major cannabinoids, i.e., CBD, THC, and CBG, but several pieces of evidence suggest that minor cannabinoids and other bioactive compounds such as terpenes also may hold potential as therapeutic agents for CRC. Data also suggest that certain combinations of cannabinoids and terpenes in C. sativa extracts can lead to a synergistic action known as the “entourage effect,” which has been linked to certain pharmacological benefits. The potential therapeutic benefits of the cannabinoids and terpenes from this plant make them key candidates for further drug development.References
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648.
- Ubeed, H.M.S.A.L.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604.
- Duggan, P.J. The Chemistry of Cannabis and Cannabinoids. Aust. J. Chem. 2021, 74, 369–387.
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and harms of medical cannabis: A scoping review of systematic reviews. Syst. Rev. 2019, 8, 320.
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 565–579.
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364.
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, C.; Prostran, M. Cannabinoids and Pain: New Insights from Old Molecules. Front. Pharmacol. 2018, 9, 1259.
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.M.; Largent-Milnes, T.M. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 1269–1297.
- McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891.
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21.
- Liu, Y.; Liu, H.-Y.; Li, S.-H.; Ma, W.; Wu, D.-T.; Li, H.-B.; Xiao, A.-P.; Liu, L.-L.; Zhu, F.; Gan, R.-Y. Cannabis sativa bioactive compounds and their extraction, separation, purification, and identification technologies: An updated review. TrAC Trends Anal. Chem. 2022, 149, 116554.
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660.
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19.
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004.
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392.
- Sommano, S.R.; Sunanta, P.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Phimolsiripol, Y.; Sringarm, K.; Ruksiriwanich, W.; Jantrawut, P.; et al. Mass Spectrometry-Based Metabolomics of Phytocannabinoids from Non-Cannabis Plant Origins. Molecules 2022, 27, 3301.
- Mnekin, L.; Ripoll, L. Topical Use of Cannabis Sativa L. Biochemicals. Cosmetics 2021, 8, 85.
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The Medicinal Natural Products of Cannabis sativa Linn.: A Review. Molecules 2022, 27, 1689.
- Barrales-Cureño, H.J.; López-Valdez, L.G.; Reyes, C.; Cetina-Alcalá, V.M.; Vasquez-García, I.; Diaz-Lira, O.F.; Herrera-Cabrera, B.E. Chemical Characteristics, Therapeutic Uses, and Legal Aspects of the Cannabinoids of Cannabis sativa: A Review. Braz. Arch. Biol. Technol. 2020, 63.
- Moreno, T.; Dyer, P.; Tallon, S. Cannabinoid Decarboxylation: A Comparative Kinetic Study. Ind. Eng. Chem. Res. 2020, 59, 20307–20315.
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314.
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines 2022, 10, 3142.
- Helcman, M.; Šmejkal, K. Biological activity of Cannabis compounds: A modern approach to the therapy of multiple diseases. Phytochem. Rev. 2022, 21, 429–470.
- Johnson, A.; Stewart, A.; El-Hakim, I.; Hamilton, T.J. Effects of super-class cannabis terpenes beta-caryophyllene and alpha-pinene on zebrafish behavioural biomarkers. Sci. Rep. 2022, 12, 17250.
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72.
- Janatová, A.; Doskočil, I.; Božik, M.; Fraňková, A.; Tlustoš, P.; Klouček, P. The chemical composition of ethanolic extracts from six genotypes of medical cannabis (Cannabis sativa L.) and their selective cytotoxic activity. Chem. Interact. 2022, 353, 109800.
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797.
- Nallathambi, R.; Mazuz, M.; Namdar, D.; Shik, M.; Namintzer, D.; Vinayaka, A.C.; Ion, A.; Faigenboim, A.; Nasser, A.; Laish, I.; et al. Identification of Synergistic Interaction between Cannabis-Derived Compounds for Cytotoxic Activity in Colorectal Cancer Cell Lines and Colon Polyps That Induces Apoptosis-Related Cell Death and Distinct Gene Expression. Cannabis Cannabinoid Res. 2018, 3, 120–135.
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23.
- Feng, P.; Zhu, L.; Jie, J.; Yang, P.; Sheng, N.; Chen, X.; Chen, X. Cannabidiol inhibits invasion and metastasis in colorectal cancer cells by reversing epithelial—Mesenchymal transition through the Wnt/β-catenin signaling pathway. J. Cancer Res. Clin. Oncol. 2022.
- Becker, W.; Alrafas, H.R.; Wilson, K.; Miranda, K.; Culpepper, C.; Chatzistamou, I.; Cai, G.; Nagarkatti, M.; Nagarkatti, P.S. Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-Activation Upstream of IL-22 Production. iScience 2020, 23, 101504.
- Khoury, M.; Cohen, I.; Bar-Sela, G. “The Two Sides of the Same Coin”—Medical Cannabis, Cannabinoids and Immunity: Pros and Cons Explained. Pharmaceutics 2022, 14, 389.
- Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer. Cancers 2021, 13, 4353.
- Lee, H.-S.; Tamia, G.; Song, H.-J.; Amarakoon, D.; Wei, C.-I.; Lee, S.-H. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int. Immunopharmacol. 2022, 108, 108865.
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642.
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic Effects of Cannabinoids on Human HT-29 Colorectal Adenocarcinoma Cells: Different Mechanisms of THC, CBD, and CB83. Int. J. Mol. Sci. 2020, 21, 5533.
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer Res. 2011, 31, 3799–3807.
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934.
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163.
- Lee, X.C.; Werner, E.; Falasca, M. Molecular Mechanism of Autophagy and Its Regulation by Cannabinoids in Cancer. Cancers 2021, 13, 1211.
- Solinas, M.; Massi, P.; Cantelmo, A.; Cattaneo, M.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L.; Noonan, D.; et al. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br. J. Pharmacol. 2012, 167, 1218–1231.
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Pathol. 2019, 28, 165–172.
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154.
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The cannabinoid δ 9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int. J. Cancer 2007, 121, 2172–2180.
- Shor, D.B.-A.; Hochman, I.; Gluck, N.; Shibolet, O.; Scapa, E. The Cytotoxic Effect of Isolated Cannabinoid Extracts on Polypoid Colorectal Tissue. Int. J. Mol. Sci. 2022, 23, 11366.
- De la Ossa, D.H.P.; Gil-Alegre, M.E.; Ligresti, A.; Aberturas, M.D.R.; Molpeceres, J.; Torres, A.I.; Di Marzo, V. Preparation and characterization of Δ9-tetrahydrocannabinol-loaded biodegradable polymeric microparticles and their antitumoral efficacy on cancer cell lines. J. Drug Target. 2013, 21, 710–718.
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1-CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632.
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067.
- Ceccarelli, I.; Fiorenzani, P.; Pessina, F.; Pinassi, J.; Aglianò, M.; Miragliotta, V.; Aloisi, A.M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020, 14, 850.
- Lucafò, M.; Curci, D.; Franzin, M.; Decorti, G.; Stocco, G. Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview from Pathophysiology to Pharmacological Prevention. Front. Pharmacol. 2021, 12, 772101.
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166.
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567.
