2. Cardio-Diabetology: The Role of Biomarkers and Inflammatory Molecules
Pathophysiological analysis of the adipovascular axis revealed the determinant role of inflammatory markers such as IL-6 and resistin in modulating cardiovascular risk in diabetic patients
[14][96]. Patients with DF syndrome associate metabolic disorders that modulate the associated cardiovascular risk. Hyperglycemia causes axonal and microvascular injury over time. In addition, in diabetic patients, hypertriglyceridemia is a known independent risk factor for amputations
[15][16][75,130].
Clinical studies in the field associate low serum levels of adiponectin and high levels of IL-6 with the development and progression of inflammatory mechanisms involved in the pathogenesis of diabetic ulcers. In diabetic patients, microalbuminuria correlates with serum IL-6 and adipocytokine levels, thus playing a dual role as a contributor to the progression of insulin resistance and the production of inflammatory cytokines
[14][96]. Besides adiposity, oxidative stress, mitochondrial dysfunction, activation of the polyol pathway or accumulation of pro-inflammatory and advanced glycation end-products are pathophysiological mechanisms underlying the cardiovascular damage associated with DF syndrome
[17][18][19][20][131,132,133,134].
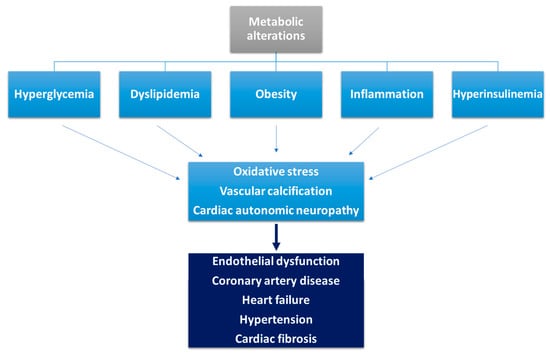
Hyperglycemia affects ischemic preconditioning, being associated with elevated serum catecholamines, the presence of a no-reflow phenomenon, increased oxidative stress, pro-thrombotic status and inflammation (
Figure 1)
[21][22][135,136]. Hyperglycemia also leads to endothelial dysfunction, with activation of various metabolic pathways mediated by protein kinase C resulting in excessive production of reactive oxygen species
[15][75]. Miric et al.
[23][137] have shown that serum xanthine oxidase activity is an independent predictor for the occurrence of diabetic peripheral neuropathy, thus contributing to the development of DF syndrome. The administration of vitamin D for 12 weeks is accompanied by an improvement in carbohydrate metabolism, which also contributes to the healing process of diabetic ulcers
[24][138]. Silent myocardial ischemia is common among diabetic patients, the presence of diabetic peripheral neuropathy being a risk factor associated with it; it has a screening role to detect patients at risk of developing an acute cardiovascular event
[25][139].
Figure 1. Pathophysiological mechanisms involved in the occurrence of cardiovascular disease in diabetic patients (adapted from
[21][135]).
In addition to IL-6, other cytokines that modulate inflammatory processes have been identified such as leptin, tumor necrosis factor-α, plasminogen activator inhibitor, uric acid or oxidized low-density lipoproteins
[26][27][28][29][103,140,141,142]. Afshinnia et al.
[29][142] identified several lipid determinants with a role in the development and progression of diabetic neuropathy such as acylcarnitines, free fatty acids, phosphatidylcholines, and lysophosphatidylcholines, the presence of which can be identified up to 10 years earlier in a high titer. Some of these lipid compounds are also responsible for the development of cardiac autonomic dysfunction in patients with type 2 DM
[30][143] and insulin resistance
[31][144].
The identification of a serum cystatin C level above 0.735 mg/L is a potential biomarker for the identification of diabetes-associated complications and is correlated with the occurrence of DF and tissue loss in patients with type 2 DM
[32][145].
In patients with type 2 diabetes, elevated homocysteine levels are associated with the presence of heart failure with a low ejection fraction and are an independent risk factor for all-cause mortality
[33][44]. The connection between cardiac autonomic neuropathy and subclinical inflammation, with multiple prognostic implications on associated cardiovascular risk and morbidity, was examined in diabetic patients, with differential results depending on the type of molecule involved
[34][35][36][146,147,148]. Heart rate variability is dependent on altered sympathetic and parasympathetic tone in young patients with DM, correlating with glycated hemoglobin values
[37][38][149,150].
While IL-18 and soluble E-selectin are associated with reduced vagal activity, adiponectin can be used as a marker of decreased sympathetic activity
[39][151]. Adiponectin has a dual, titer-dependent role, being both a cardioprotective hormone with an anti-inflammatory role and a marker of high cardiovascular risk through its association with an assessment of the risk of death
[40][41][152,153]. Adiponectin also prevents cardiac remodeling after myocardial infarction and inhibits the action of the sympathetic nervous system on this pathophysiological process, also having a potential therapeutic role in these patients
[42][154]. Zhu et al.
[43][155] demonstrated that elevated serum adiponectin levels are an independent predictor of atrial fibrillation in women and men under 65 years old.
Peripheral somatic neuropathy contributes to the development of medial arterial calcification
[44][156]. In diabetic patients, it plays a role in activating the receptor activator of nuclear factor kappa B ligand/osteoprotegerin signaling pathway that modulates the pathophysiological processes involved in the calcification of smooth muscle cells in the coronary arteries and peripheral limbs
[16][130]. Advanced glycation end-products also contribute to the production of vascular calcification and, thus, to the progression of atherosclerotic lesions in diabetic patients
[45][46][157,158].
Serum B-type natriuretic peptide levels correlate with the risk of onset and progression of diabetic neuropathy, but further clinical research is needed to unravel any pathophysiological implications. Data in the literature to date attest to the role of this cardiovascular biomarker in identifying and assessing the extent of neuropathic damage in diabetic patients. Based on the role of BNP to assess the presence of systolic and diastolic dysfunction as well as to assess the prognosis of patients with heart failure, the identification of a connection with DFS is of great clinical importance, as it can also assess the cardiovascular risk of patients without previously known cardiac pathology
[47][48][49][159,160,161]. Yan et al.
[50][162] demonstrated that serum BNP levels were higher in patients with type 2 DM and diabetic neuropathy compared to a cohort without microvascular damage (
p = 0.001), while positively correlating with systolic blood pressure, serum creatinine, prevalence of diabetic ulcers (
p = 0.039) and vibration perception threshold values (
p = 0.021). Identification of a BNP value above 15.18 pg/mL has a sensitivity of 78.7% and a specificity of 48.2% in assessing the presence of diabetic neuropathy.
A recently published meta-analysis by Ramzi et al.
[51][163] highlights that the presence of a serum N-terminal prohormone brain natriuretic peptide (NT-proBNP) level above 225 pg/mL correlates with a high risk of death from any cause among diabetic patients, while serum levels above 100 pg/mL are predictive of the occurrence of acute cardiovascular events. This biomarker, along with IL-6 and resistin, correlates with the presence of left ventricular dysfunction and left ventricular hypertrophy and can thus be used as non-invasive biomarkers for assessing cardiovascular risk in patients with DM
[52][164].
3. Diabetic Foot Syndrome and Diabetic Cardiomyopathy
3.1. The Role of Artificial Intelligence in Assessing CVD Risk in DM
The applications of artificial intelligence in the medical field are becoming more and more extensive, being able nowadays to generate various models of cardiovascular risk assessment with both therapeutic and prognostic roles
[53][54][205,206]. Imaging assessment of the ulcerative lesion is essential in order to identify an associated infection
[55][207] and to provide accurate epidemiological data on the prevalence of DM
[56][208]. Clinical studies in the literature have demonstrated that diabetic foot infections act as a cardiovascular marker based on a directly proportional correlation between the severity of infection and atherosclerotic lesions
[15][57][75,209].
Machine Learning (ML) and Deep Learning (DL) algorithms have various applications in cardiology and diabetology
[58][59][210,211], having both diagnostic and prognostic roles by identifying predictors associated with cardiovascular risk
[60][61][62][212,213,214]. Technologies using artificial intelligence are based on the analysis of complex databases, and current data on diabetes-related cardiovascular risk assessment show non-linear connections between input predictors and the obtained risk. The advantage of using the aforementioned algorithms is obtaining intrinsic relationships between several predictors used simultaneously
[63][64][65][215,216,217]. To date, several algorithms have been developed to assess associated cardiovascular risk
[66][67][68][218,219,220], one such example being the one proposed by Jamthikar et al.
[69][221] which, using carotid vascular Doppler assessment and the presence of traditional cardiovascular risk factors, can assess associated cardiovascular risk with superior accuracy to traditional methods of calculation. Based on the socio-economic importance of the complications associated with DFS, artificial intelligence algorithms have been developed to screen diabetic patients to identify risk factors for the development of ulcers using different optical sensors
[70][71][72][73][222,223,224,225].
3.2. Predictive Risk Models for CVD Events in DM
The development of cardiovascular risk prediction models in diabetic patients is an extremely valuable clinical tool in the daily practice of cardio-diabetology
[74][226]. Most risk prediction models to date are based on data from the Framingham Heart Study, in which diabetes is included as a risk factor
[75][76][227,228]. This prediction model can be used among diabetic patients due to the underestimation of the associated cardiovascular risk, which has required the development of new prediction tools for this population
[74][77][226,229]. The United Kingdom Prospective Diabetes Study and the ADVANCE study
[78][79][80][230,231,232] identified a number of predictors which were demographic (age), associated with DM (time since diagnosis, glycosylated hemoglobin value) or derived from major cardiovascular risk factors.
Similarly, Mu et al.
[81][233] developed an algorithm to predict the risk of an acute fatal or non-fatal cardiovascular event (especially acute myocardial infarction or stroke) in the next 10 years in patients with type 2 diabetes. Thus, the QRESEARCH risk estimator version 3 is superior to the Framingham risk score by using a much larger number of clinical–paraclinical parameters and has a much higher predictability among diabetic patients
[82][83][234,235]. Based on the observation that approximately 50% of patients with cardiovascular disease do not associate with traditional cardiovascular risk factors, some clinical prediction models integrate biomarkers, comorbidities, polygenic-based scores
[84][85][236,237] or metabolomic patterns
[86][87][88][238,239,240].
Although various such prediction models have been developed, methodological issues, high risk of bias and lack of clinical validation studies are some of the main drawbacks identified that reinforce the need for extensive clinical research on large cohorts of patients before introduction into clinical practice
[89][241].
3.3. Novel Therapeutic Targets
Advances in technology have enabled advanced clinical research, and various signaling pathways are currently being investigated for future use in the development of targeted therapeutic molecules
[90][180]. P38 kinases are considered valuable and promising therapeutic targets in preventing the onset or progression of diabetic cardiomyopathy based on p38-MAPK-generated inhibition that resulted in improved inflammatory status and systolic function in previous animal studies of diabetic mice
[91][242]. The use of phosphodiesterase type 5 inhibitors may prevent hyperglycemia-induced changes in cardiomyocyte gene expression, thereby counteracting the increased cyclic adenosine 5-monophosphate-responsive element modulator
[92][243].
Modulation of nuclear factor erythroid 2-related factor 2 activity is associated with decreased inflammation and lipid accumulation or prevention of the development of fibrosis secondary to DM, thus representing a potential therapeutic target in patients with diabetic cardiomyopathy
[93][244]. The use of exosomes developed from heat shock protein 20 has been shown in animal studies to modulate the secretory activity of cardiomyocytes
[94][245]. microRNAs and the correction of intestinal dysbiosis interfere with the pathophysiological processes of diabetic cardiomyopathy
[95][246]. In a previous clinical study, Katare et al.
[96][247] demonstrated that anti-miR1 induces survival signals in cardiac progenitor cells or cardiomyocytes subjected to permanent hyperglycemic status.
Gene therapy is a very promising future research direction in relation to DM and DFS. To date, pre-clinical research with murine leukemia virus-1 (PIM-1) via cardiotropic serotype-9 adeno-associated virus (AAV) which increased PIM-1 expression and cardiac phosphoinositide 3-kinase using AAV6 has demonstrated in vitro prevention of cardiac apoptosis, fibrosis or development of heart failure and increased LV systolic function in diabetic mice
[96][97][98][176,178,247].
The Charcot DF is frequently encountered in patients with DM, being one of the most disabling and severe complications
[99][248]. The pathophysiology is complex and multifactorial, involving numerous signaling pathways and molecules that mediate diverse, interconnected processes that over time result in osteolysis and ultimately bone destruction
[100][101][249,250]. One such example is the receptor activator of the NF-κB ligand receptor activator of NF-κB osteoprotegerin (RANKL-RANK-OPG) pathway which modulates inflammatory processes, its overexpression and activation being accompanied by increased osteoclast activity and osteolysis
[102][251]. Taking into account the role of this signaling pathway in bone remodeling in patients with DFS, several therapeutic agents have been developed to act at different levels of the signaling pathway with the aim of halting the disabling progression of Charcot DF
[102][251]. One such example is the administration of anti-RANKL monoclonal antibodies such as Denosumab, which—although it has, so far, been studied in small groups of patients—has encouraging results that justify further research in larger groups of patients
[103][103][104][105][252,252,253,254].
In terms of wound healing, previous clinical and animal studies have demonstrated that ulcer-associated pro-inflammatory status, lack of ischemia correction and lesion maturation are therapeutic targets associated with improved wound healing in patients with DFS
[106][255]. The neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are two easily measurable, reproducible inflammatory biomarkers with prognostic value in both the onset and progression of DFS. Among patients with DF-associated infections, elevated values of these biomarkers correlate with the occurrence of osteomyelitis, the need for amputation or septic complications
[107][108][256,257].