Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Tomasz Swebocki.
Deep eutectic solvents (DESs), considered as one of the greenest families of solvents, are used in many fields, such as organic synthesis, (bio)catalysis, electrochemistry, and (bio)medicine.
- deep eutectic solvents
- organic acids
- drug delivery
- enhancers
1. Brief Background and State-of-the-Art
Deep eutectic solvents (DESs) are a fascinating group of substances introduced to a broader scientific audience in 2003 by Abbott et al. [1]. Since then, DESs have brought more and more interest with observable ‘boom’ over recent years (Figure 1). Their medical-related applications contribute to 25–50% of the papers published in the last eight years. A similar trend was also observed for the number of works published in DESs in biomedicine, which mostly covers similar applications. Due to their negligible adverse effect on the environment, chemical composition and low cytotoxicity, they are considered a family of green solvents [2]. DESs have found their use in many fields of chemistry, such as catalysis [3], metal processing [4], synthesis of nanoparticles [5], electrochemistry [6,7][6][7] etc. Their application versatility arises from the number of possible combinations of parent substances (PS) needed for their syntheses. Unsurprisingly, they have also been investigated in various aspects of biomedicine, such as anti-bacterial treatment, drug delivery and solubilization.
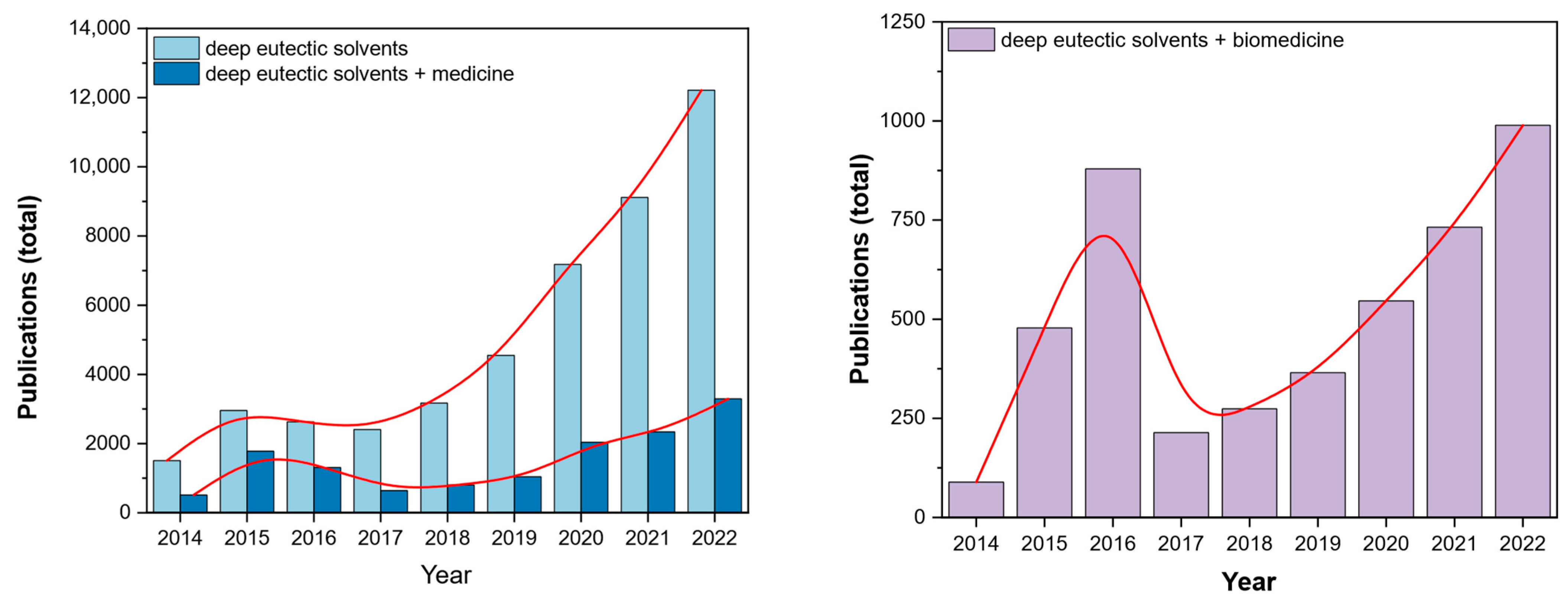
Figure 1. Number of papers published over the 2014–2022 period covering the interest in the field over the recent years (keywords: deep eutectic solvents, deep eutectic solvents + medicine, deep eutectic solvents + biomedicine, according to Digital Science & Research Solutions, Inc., London, UK).
The application of DESs has already been covered in many review articles [8[8][9][10][11][12],9,10,11,12], including two crucial applications in medicine—drug delivery enhancement and treatment of bacterial infections—for instance, El. Achkar et al. [12] summarized the different routes for synthesising DESs and the most relevant parameters controlling their formation and physicochemical properties. In a recent extended review article, Hansen et al. [10], after a brief discussion of the various application ranging from metallurgy to pharmaceutical and biomedical fields of DESs, gave a deep and detailed discussion of the literature results to understand the microscopic mechanisms governing the structure-property relationships. In the review report by Liu et al. [11], the importance of natural deep eutectic solvents (NADESs) in natural products and biological systems was highlighted through various applications, such as extraction and chromatographic media. Zainal-Abidin et al. [13] and Huang et al. [14] described respectively the application of DES in drug discovery and delivery systems and as active pharmaceutical ingredient (API) delivery systems in the treatment of metabolic diseases (cancer, diabetes, and atherosclerosis).
Moreover, using DES might be a better strategy than a straightforward transformation of APIs into ionic liquids (IL). First of all, not all of the APIs can be transformed into classic ILs—the targeted API should be in the ionic form that allows for the formation of IL. Common cations include imidazole derivatives or other N-heterocyclic compounds. Imidazole can sometimes be found in drugs (mainly in antifungal therapy [15]), but it is not a common structural motif. In addition, the size of the molecule is crucial for the formation of ILs. It is possible to form API-IL [16], but this usually applies to transforming small compounds, excluding biomacromolecules like peptides, proteins and modified DNA. Moreover, even though the development and medical-application optimization of ILs is ongoing, their cytotoxicity and environmental impact are still questioned [17,18,19][17][18][19]. Adding to it their high production cost and not-so-straightforward synthesis, it seems reasonable to look for alternative strategies for API delivery.
2. Physicochemistry of Deep Eutectic Solvents (DESs)
Deep eutectic solvents are mixtures of hydrogen bond donors (HBDs) and acceptors (HBAs) (Lewis and Brønsted acids and bases), which are characterized by the presence of the eutectic point. Eutectic point is associated with a specific ratio of parent substances (PSs) at which their mixture exhibits a much lower melting point than the isolated PSs. In the case of DESs, the depression of the melting point is much deeper than for common eutectics (Figure 2); hence, the term “deep eutectic”. An example of that phenomenon can be observed in the common DES called reline—a mixture of choline chloride (ChCl) and urea (U) in a molar ratio of 1:2, which was first discovered by Abbott et al. [1]. While the melting points of ChCl and U are 302–305 °C and 133–135 °C, respectively, the melting temperature of reline is ~12 °C.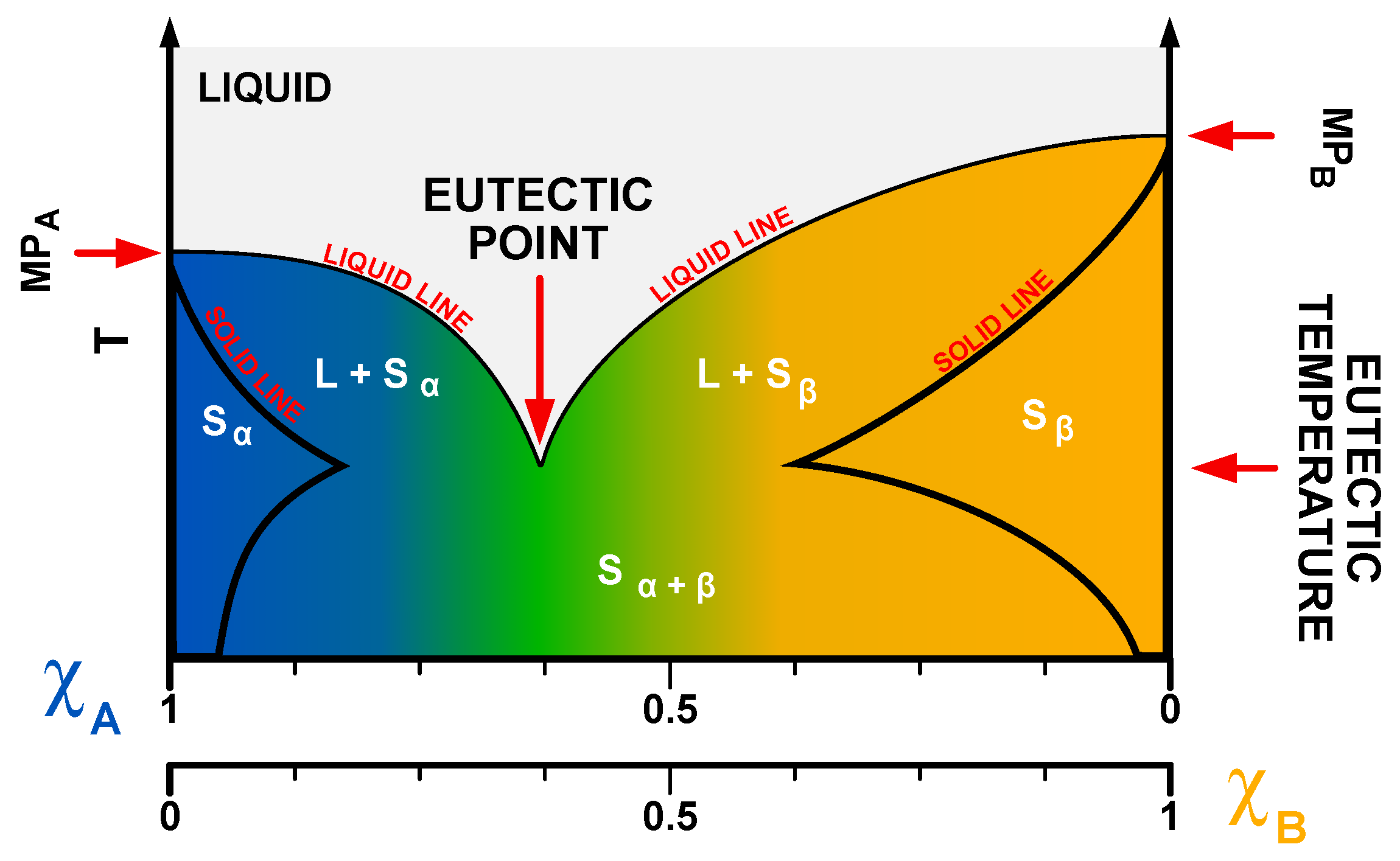
Figure 2. Phase diagram of the binary DESs. Note the presence of a deep eutectic point. S and L—solid and liquid phase, α and β—phase names, χ—molar fraction of substance A and B.
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 9, 70–71.
- Vian, M.; Breil, C.; Vernes, L.; Chaabani, E.; Chemat, F. Green Solvents for Sample Preparation in Analytical Chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 44–48.
- Ünlü, A.E.; Arıkaya, A.; Takaç, S. Use of Deep Eutectic Solvents as Catalyst: A Mini-Review. Green Process. Synth. 2019, 8, 355–372.
- Smith, E.L. Deep Eutectic Solvents (DESs) and the Metal Finishing Industry: Where Are They Now? Trans. Inst. Met. Finish. 2013, 91, 241–248.
- Liao, H.-G.; Jiang, Y.-X.; Zhou, Z.-Y.; Chen, S.-P.; Sun, S.-G. Shape-Controlled Synthesis of Gold Nanoparticles in Deep Eutectic Solvents for Studies of Structure–Functionality Relationships in Electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103.
- Figueiredo, M.; Gomes, C.; Costa, R.; Martins, A.; Pereira, C.M.; Silva, F. Differential Capacity of a Deep Eutectic Solvent Based on Choline Chloride and Glycerol on Solid Electrodes. Electrochim. Acta 2009, 54, 2630–2634.
- Costa, R.; Figueiredo, M.; Pereira, C.M.; Silva, F. Electrochemical Double Layer at the Interfaces of Hg/Choline Chloride Based Solvents. Electrochim. Acta 2010, 55, 8916–8920.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Cunha, S.C.; Fernandes, J.O. Extraction Techniques with Deep Eutectic Solvents. Trends Anal. Chem. 2018, 105, 225–239.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285.
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690.
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408.
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging Frontiers of Deep Eutectic Solvents in Drug Discovery and Drug Delivery Systems. J. Control. Release 2019, 316, 168–195.
- Huang, C.; Chen, X.; Wei, C.; Wang, H.; Gao, H. Deep Eutectic Solvents as Active Pharmaceutical Ingredient Delivery Systems in the Treatment of Metabolic Related Diseases. Front. Pharmacol. 2021, 12, 794939.
- Liu, C.; Shi, C.; Mao, F.; Xu, Y.; Liu, J.; Wei, B.; Zhu, J.; Xiang, M.; Li, J. Discovery of New Imidazole Derivatives Containing the 2,4-Dienone Motif with Broad-Spectrum Antifungal and Antibacterial Activity. Molecules 2014, 19, 15653–15672.
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active Pharmaceutical Ingredients (APIs) in Ionic Liquids: An Effective Approach for API Physiochemical Parameter Optimization. Drug Discov. Today 2022, 27, 2415–2424.
- Musiał, M.; Zorębski, E.; Malarz, K.; Kuczak, M.; Mrozek-Wilczkiewicz, A.; Jacquemin, J.; Dzida, M. Cytotoxicity of Ionic Liquids on Normal Human Dermal Fibroblasts in the Context of Their Present and Future Applications. ACS Sustain. Chem. Eng. 2021, 9, 7649–7657.
- Shamsuri, A.A. Ionic Liquids: Preparations and Limitations. Makara J. Sci. 2011, 14, 101–106.
- Hu, L.-X.; Xiong, Q.; Shi, W.-J.; Huang, G.-Y.; Liu, Y.-S.; Ying, G.-G. New Insight into the Negative Impact of Imidazolium-Based Ionic Liquid Cl on Hela Cells: From Membrane Damage to Biochemical Alterations. Ecotoxicol. Environ. Saf. 2021, 208, 111629.
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Yadav, A.; Trivedi, S.; Rai, R.; Pandey, S. Densities and Dynamic Viscosities of (Choline Chloride+glycerol) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range (283.15–363.15)K. Fluid Phase Equilib. 2014, 367, 135–142.
- van den Bruinhorst, A.; Costa Gomes, M. Is There Depth to Eutectic Solvents? Curr. Opin. Green Sustain. Chem. 2022, 37, 100659.
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, Hydrogen Bonding and Rheology in Choline Chloride Deep Eutectic Solvents as a Function of the Hydrogen Bond Donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306.
- Araujo, C.F.; Coutinho, J.A.P.; Nolasco, M.M.; Parker, S.F.; Ribeiro-Claro, P.J.A.; Rudić, S.; Soares, B.I.G.; Vaz, P.D. Inelastic Neutron Scattering Study of Reline: Shedding Light on the Hydrogen Bonding Network of Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2017, 19, 17998–18009.
More
