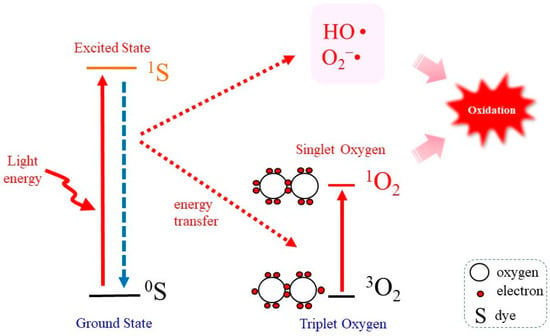
Energy transfer to ground state triplet molecular oxygen results in the generation of singlet molecular oxygen (1O2), which has potent oxidizing ability. Irradiation of light, notably ultraviolet A, to a photosensitizing molecule results in the generation of 1O2, which is thought to play a role in causing skin damage and aging. It should also be noted that 1O2 is a dominant tumoricidal component that is generated during the photodynamic therapy (PDT). While type II photodynamic action generates not only 1O2 but also other reactive species, endoperoxides release pure 1O2 upon mild exposure to heat and, hence, are considered to be beneficial compounds for research purposes. Concerning target molecules, 1O2 preferentially reacts with unsaturated fatty acids to produce lipid peroxidation. Enzymes that contain a reactive cysteine group at the catalytic center are vulnerable to 1O2 exposure. Guanine base in nucleic acids is also susceptible to oxidative modification, and cells carrying DNA with oxidized guanine units may experience mutations.
- photodynamic therapy
- ultraviolet
- endoperoxides
1. Introduction
2. Properties of 1O2 as a Potent Oxidant
3. Chemical Probes for Detecting 1O2
4. Photodynamic Reaction as a 1O2 -Generating System
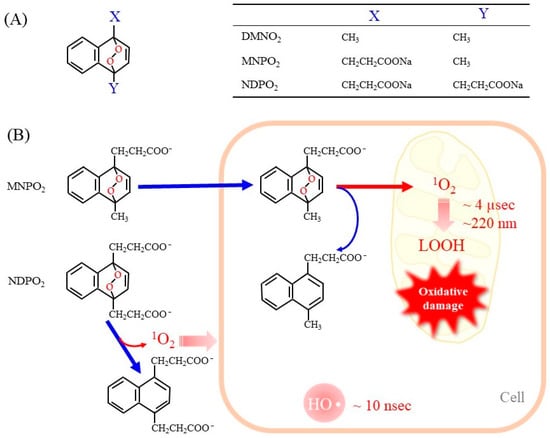
5. Endoperoxides as Donor Compounds for Generating Pure 1O2
6. Natural or Synthetic 1O2-Scavenging Compounds
References
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112.
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501.
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine Meets Fenton Chemistry. Chem Rev. 2021, 121, 1981–2019.
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628.
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10.
- Petrou, A.L.; Terzidaki, A. A meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases. Biochem. J. 2017, 474, 2713–2731.
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086.
- Murotomi, K.; Umeno, A.; Shichiri, M.; Tanito, M.; Yoshida, Y. Significance of Singlet Oxygen Molecule in Pathologies. Int. J. Mol. Sci. 2023, 24, 2739.
- Ahmad, N.; Mukhtar, H. Mechanism of photodynamic therapy-induced cell death. Methods Enzymol. 2000, 319, 342–358.
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802.
- Garcia-Diaz, M.; Huang, Y.Y.; Hamblin, M.R. Use of fluorescent probes for ROS to tease apart Type I and Type II photochemical pathways in photodynamic therapy. Methods 2016, 109, 158–166.
- Pierlot, C.; Aubry, J.M.; Briviba, K.; Sies, H.; Di Mascio, P. Naphthalene endoperoxides as generators of singlet oxygen in biological media. Methods Enzymol. 2000, 319, 3–20.
- Krutmann, J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000, 23 (Suppl. S1), S22–S26.
- Tyrrell, R.M. Solar ultraviolet A radiation: An oxidizing skin carcinogen that activates heme oxygenase-1. Antioxid. Redox Signal. 2004, 6, 835–840.
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883.
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15.
- Zhuang, S.; Kochevar, I.E. Singlet oxygen-induced activation of Akt/protein kinase B is independent of growth factor receptors. Photochem. Photobiol. 2003, 78, 361–371.
- Le Panse, R.; Dubertret, L.; Coulomb, B. p38 mitogen-activated protein kinase activation by ultraviolet A radiation in human dermal fibroblasts. Photochem. Photobiol. 2003, 78, 168–174.
- Morita, A.; Werfel, T.; Stege, H.; Ahrens, C.; Karmann, K.; Grewe, M.; Grether-Beck, S.; Ruzicka, T.; Kapp, A.; Klotz, L.O.; et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J. Exp. Med. 1997, 186, 1763–1768.
- Klotz, L.O.; Kröncke, K.D.; Sies, H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem. Photobiol. Sci. 2003, 2, 88–94.
- Novikova, I.N.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V.; Abramov, A.Y. Laser-induced singlet oxygen selectively triggers oscillatory mitochondrial permeability transition and apoptosis in melanoma cell lines. Life Sci. 2022, 304, 120720.
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072.
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421.
- Homma, T.; Kobayashi, S.; Fujii, J. Induction of ferroptosis by singlet oxygen generated from naphthalene endoperoxide. Biochem. Biophys. Res. Commun. 2019, 518, 519–525.
- Meng, X.; Deng, J.; Liu, F.; Guo, T.; Liu, M.; Dai, P.; Fan, A.; Wang, Z.; Zhao, Y. Triggered All-Active Metal Organic Framework: Ferroptosis Machinery Contributes to the Apoptotic Photodynamic Antitumor Therapy. Nano Lett. 2019, 19, 7866–7876.
- Wefers, H.; Sies, H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur. J. Biochem. 1983, 137, 29–36.
- Di Mascio, P.; Briviba, K.; Sasaki, S.T.; Catalani, L.H.; Medeiros, M.H.; Bechara, E.J.; Sies, H. The reaction of peroxynitrite with tert-butyl hydroperoxide produces singlet molecular oxygen. Biol. Chem. 1997, 378, 1071–1074.
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B. 2014, 139, 24–33.
- Redmond, R.W.; Kochevar, I.E. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006, 82, 1178–1186.
- Jiménez-Banzo, A.; Sagristà, M.L.; Mora, M.; Nonell, S. Kinetics of singlet oxygen photosensitization in human skin fibroblasts. Free Radic. Biol. Med. 2008, 44, 1926–1934.
- Driever, S.M.; Fryer, M.J.; Mullineaux, P.M.; Baker, N.R. Imaging of reactive oxygen species in vivo. Methods Mol. Biol. 2009, 479, 109–116.
- Pedersen, S.K.; Holmehave, J.; Blaikie, F.H.; Gollmer, A.; Breitenbach, T.; Jensen, H.H.; Ogilby, P.R. Aarhus sensor green: A fluorescent probe for singlet oxygen. J. Org. Chem. 2014, 79, 3079–3087.
- Tang, C.Y.; Wu, F.Y.; Yang, M.K.; Guo, Y.M.; Lu, G.H.; Yang, Y.H. A Classic Near-Infrared Probe Indocyanine Green for Detecting Singlet Oxygen. Int. J. Mol. Sci. 2016, 17, 219.
- Ruiz-González, R.; Bresolí-Obach, R.; Gulías, Ò.; Agut, M.; Savoie, H.; Boyle, R.W.; Nonell, S.; Giuntini, F. NanoSOSG: A Nanostructured Fluorescent Probe for the Detection of Intracellular Singlet Oxygen. Angew Chem. Int. Ed. Engl. 2017, 56, 2885–2888.
- Chen, B.; Yang, Y.; Wang, Y.; Yan, Y.; Wang, Z.; Yin, Q.; Zhang, Q.; Wang, Y. ACS Precise Monitoring of Singlet Oxygen in Specific Endocytic Organelles by Super-pH-Resolved Nanosensors. Appl. Mater. Interfaces 2021, 13, 18533–18544.
- Nath, P.; Hamadna, S.S.; Karamchand, L.; Foster, J.; Kopelman, R.; Amar, J.G.; Ray, A. Intracellular detection of singlet oxygen using fluorescent nanosensors. Analyst 2021, 146, 3933–3941.
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715.
- Murotomi, K.; Umeno, A.; Sugino, S.; Yoshida, Y. Quantitative kinetics of intracellular singlet oxygen generation using a fluorescence probe. Sci. Rep. 2020, 10, 10616.
- Zhao, H.; Takano, Y.; Sasikumar, D.; Miyatake, Y.; Biju, V. Excitation-Wavelength-Dependent Functionalities of Temporally Controlled Sensing and Generation of Singlet Oxygen by a Photoexcited State Engineered Rhodamine 6G-Anthracene Conjugate. Chemistry 2022, 28, e202202014.
- Sasikumar, D.; Takano, Y.; Zhao, H.; Kohara, R.; Hamada, M.; Kobori, Y.; Biju, V. Caging and photo-triggered uncaging of singlet oxygen by excited state engineering of electron donor-acceptor-linked molecular sensors. Sci. Rep. 2022, 12, 11371.
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919.
- Baptistal, M.S.; Cadet, J.; Greer, A.; Thomas, A.H. Photosensitization Reactions of Biomolecules: Definition, Targets and Mechanisms. Photochem. Photobiol. 2021, 97, 1456–1483.
- Girotti, A.W.; Kriska, T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid. Redox Signal. 2004, 6, 301–310.
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387.
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485.
- Kochevar, I.E.; Redmond, R.W. Photosensitized production of singlet oxygen. Methods Enzymol. 2000, 319, 20–28.
- Jablonski, A. Efficiency of anti-stokes fluorescence in dyes. Nature 1933, 131, 839–840.
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224.
- Wei, L.; Zhang, Z.; Kumar, A.; Banerjee, S.; Huang, H. Endoperoxides Compounds for Highly Efficient Cancer Treatment under Hypoxia. Chemistry 2022, 28, e202202233.
- Ravanat, J.L.; Di Mascio, P.; Martinez, G.R.; Medeiros, M.H.; Cadet, J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2000, 275, 40601–40604.
- Ravanat, J.L.; Douki, T.; Duez, P.; Gremaud, E.; Herbert, K.; Hofer, T.; Lasserre, L.; Saint-Pierre, C.; Favier, A.; Cadet, J. Cellular background level of 8-oxo-7,8-dihydro-2’-deoxyguanosine: An isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis 2002, 23, 1911–1918.
- Otsu, K.; Sato, K.; Ikeda, Y.; Imai, H.; Nakagawa, Y.; Ohba, Y.; Fujii, J. An abortive apoptotic pathway induced by singlet oxygen is due to the suppression of caspase activation. Biochem. J. 2005, 389, 197–206.
- Lu, F.; Pan, L.; Wu, T.; Pan, W.; Gao, W.; Li, N.; Tang, B. An endoperoxide-containing covalent organic framework as a singlet oxygen reservoir for cancer therapy. Chem. Commun. 2022, 58, 11013–11016.
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391.
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626.
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.; Mukai, K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food Chem. 2010, 58, 9967–9978.
- Foote, C.S.; Chang, Y.C.; Denny, R.W. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J. Am. Chem. Soc. 1970, 92, 5216–5218.
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids-A Review. Antioxidants 2018, 7, 5.
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538.
- Garmyn, M.; Ribaya-Mercado, J.D.; Russel, R.M.; Bhawan, J.; Gilchrest, B.A. Effect of beta-carotene supplementation on the human sunburn reaction. Exp. Dermatol. 1995, 4, 104–111.
- Stahl, W.; Sies, H. Carotenoids and protection against solar UV radiation. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 291–296.
- Sies, H.; Stahl, W. Carotenoids and UV protection. Photochem. Photobiol. Sci. 2004, 3, 749–752.
- Ozkan, G.; Günal-Köroğlu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.M.; Al-Omari, B.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023, 161, 114428.
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448.
- Sommani, P.; Arai, T.; Yamashita, K.; Miyoshi, T.; Mori, H.; Sasada, M.; Makino, K. Effects of edaravone on singlet oxygen released from activated human neutrophils. J. Pharmacol. Sci. 2007, 103, 117–120.
- Nishinaka, Y.; Mori, H.; Endo, N.; Miyoshi, T.; Yamashita, K.; Adachi, S.; Arai, T. Edaravone directly reacts with singlet oxygen and protects cells from attack. Life Sci. 2010, 86, 808–813.
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Inhibition of plasma lipid oxidation induced by peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen by clinical drugs. Bioorg. Med. Chem. Lett. 2016, 26, 5411–5417.
