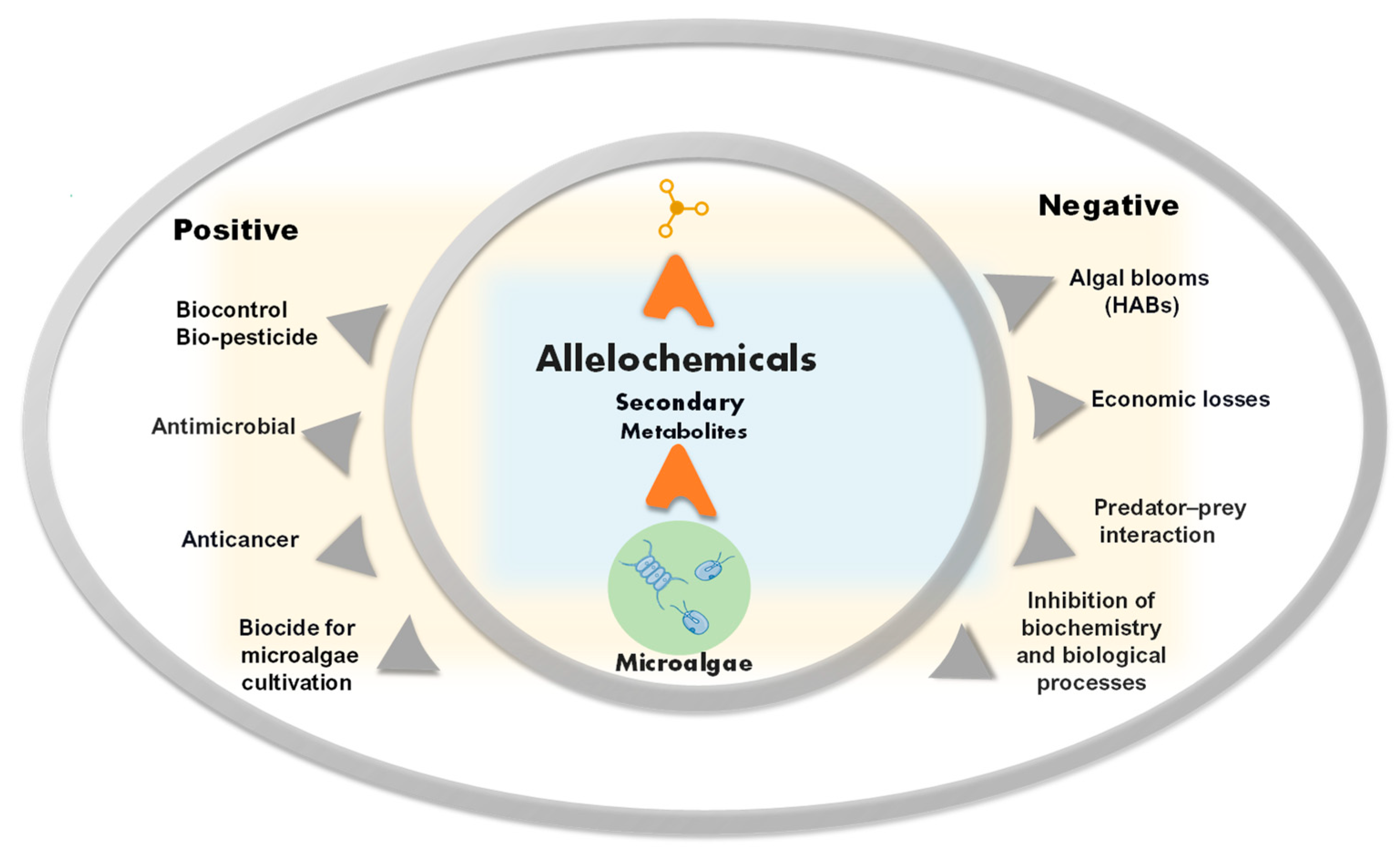
Allelochemicals are toxic secondary metabolites produced by plants, microalgae, bacteria, and fungi that influence other organisms. The bioactivity of allelochemicals and their toxic or beneficial effects have been the focus of research in medicine and agriculture, and for their anticancer and antimicrobial properties. Microalgae are the source of a remarkable diversity of biologically active compounds, which can be involved in allelopathic interactions. The main chemical classes of microalgal allelochemicals are alkaloids, fatty acids and derivatives, polyketides, peptides, phenolics, and terpenoids. In the environment, these molecules are secreted by microalgae for communication, defence, and adaptation purposes.
- allelopathy
- allelochemical
- microalgae
1. Introduction
2. Allelopathy in Microalgae
As mentioned previously, the term allelopathy was originally used to describe biomolecules produced by plants. However, it is now openly used with other organisms and has been studied in microalgae [19,20][16][17]. Microalgae organisms produce numerous secondary metabolites, including fatty acids, alkaloids, amino acids, and peptides, which are investigated and characterized as natural products by chemists and described as novel bio compounds. In the environment, these molecules are secreted for communication, defence, and adaptation purposes [21][18]. More knowledge is needed in order to understand the complexity of these bioactive chemicals and their interaction with and effect on ecosystems [19,22][16][19]. A recent review paper showed that Microcystis, Nodularia, Karenia, Alexandrium (A. catenella, A. fundyense, and others species), Skeletonema, Chlorella (C. vulgaris, C. sorokiniana, C. ellipsoidea), Chlamydomonas, and Dunaliella displayed allelopathic activity against other microalgae and cyanobacteria [23][20]. One strategy to study the allelopathic effects of different microalgae agents is to analyse the biochemicals present in mono- and co-cultures [24][21]. Wang et al. [24][21] observed that the dinoflagellate Scrippsiella acuminata had its growth significantly inhibited when in co-cultivation and/or in the presence of exudates (cell-free and sonicated-cell filtrates) from three diatoms (Chaetoceros curvisetus, Phaeodactylum tricornutum and Skeletonema dohrnii), suggesting the diatoms developed significant competitive advantage over other antagonists. Furthermore, the authors detected several types of volatile organic compounds (VOCs) in the sonicated cell-free filtrates. Polyunsaturated aldehydes (PUAs) were found in all three diatoms that showed inhibitory effects on the growth of S. acuminata and the diatoms themselves. Generally, the production of microalgae VOCs is influenced by environmental conditions, such as nutrient availability, temperature, and light incidence [25,26][22][23]. Studies show that allelochemicals of microalgae have either a positive or negative impact on other organisms. Those which can cause inhibitory effects are suitable for application as biopesticides, and as a new source of antimicrobials or bio-herbicide agents [19[16][20],23], especially because these biocompounds present increased target specificity [27][24]. The liberation of species-specific allelochemicals is desired in agriculture and is an important focus for future studies because they can be responsible for modulating the ecosystem under favourable conditions to an allelopathic microalgae. An example of this was observed in a study where, by alternating the nutrients conditions and balance of nitrogen and phosphorus sources, the allelopathic interaction between strains of Anabaena spp. and Microcystis spp. seemed to control their relative abundance and their dominance in that habitat [28][25]. An interesting point to remark upon here is that one of several new applications of microalgae is as a bioenergy source and an alternative to fossil fuels [29,30,31][26][27][28]. Nevertheless, one of the elements that industry must be alert to is the accumulation of allelopathic molecules released into the water by microalgae themselves. These compounds, when in high concentrations due to high biomass, along with other biomolecules can trigger positive or negative effects on other cells, leading to auto-stimulation or self-inhibition [32,33,34][29][30][31]. Lu and colleagues (2020) described studies that focused on water reuse for microalgae cultivation, and stated that several factors, including allelopathy mechanisms, cause cell damage, aggregation, and programmed cell death (PCD) (negative effects) or, on the other hand, the liberation of growth regulators and removal of growth inhibitors (positive effects). Bacterial agents can have a significant influence on allelopathy affecting microalgae biomass tanks [33,35][30][32]. Some allelopathic molecules have been characterized in the scientific literature as having a role in algal community growth regulation. Satake et al. [36][33] described a 19 membered ring-structured molecule called alexandrolide, which was isolated from dinoflagellate Alexandrium catenella and inhibited diatoms (Skeletonema costatum and Chattonella antiqua) but did not inhibit other dinoflagellates. Marennine, a blue-green polyphenolic pigment produced by diatom Haslea ostrearia, was described as being responsible for inhibiting the growth of other diatom species and self-stimulating the producer species in oyster-ponds [37][34]. By elucidating the properties of these compounds, more studies can be performed to apply these chemicals for industrial and human purposes, such as in additives in agricultural technology [38][35].3. Allelochemicals from Microalgae
Microalgae are the source of a remarkable diversity of biologically active compounds, which can be involved in allelopathic interactions. The main chemical classes of microalgal allelochemicals are: alkaloids, fatty acids and derivatives, polyketides, peptides, phenolics, and terpenoids.3.1. Alkaloids
Alkaloids are a prominent class of allelochemicals with biocidal properties. They consist of nitrogen-containing heterocyclic substances, in which the nitrogen atom is usually derived from an amino acid [39,40][36][37]. Alkaloids have been reported mainly in cyanobacteria and dinoflagellates. In the former, indole alkaloids are the most frequent. For instance, 12-epi-hapalindole E isonitrile (Figure 2A; 1), from Fischerella sp., is an indole alkaloid with activity against green microalgae and other microorganisms [41][38]. Another example is the toxin calothrixin A (Figure 2A; 2) from Calothrix sp [41][38].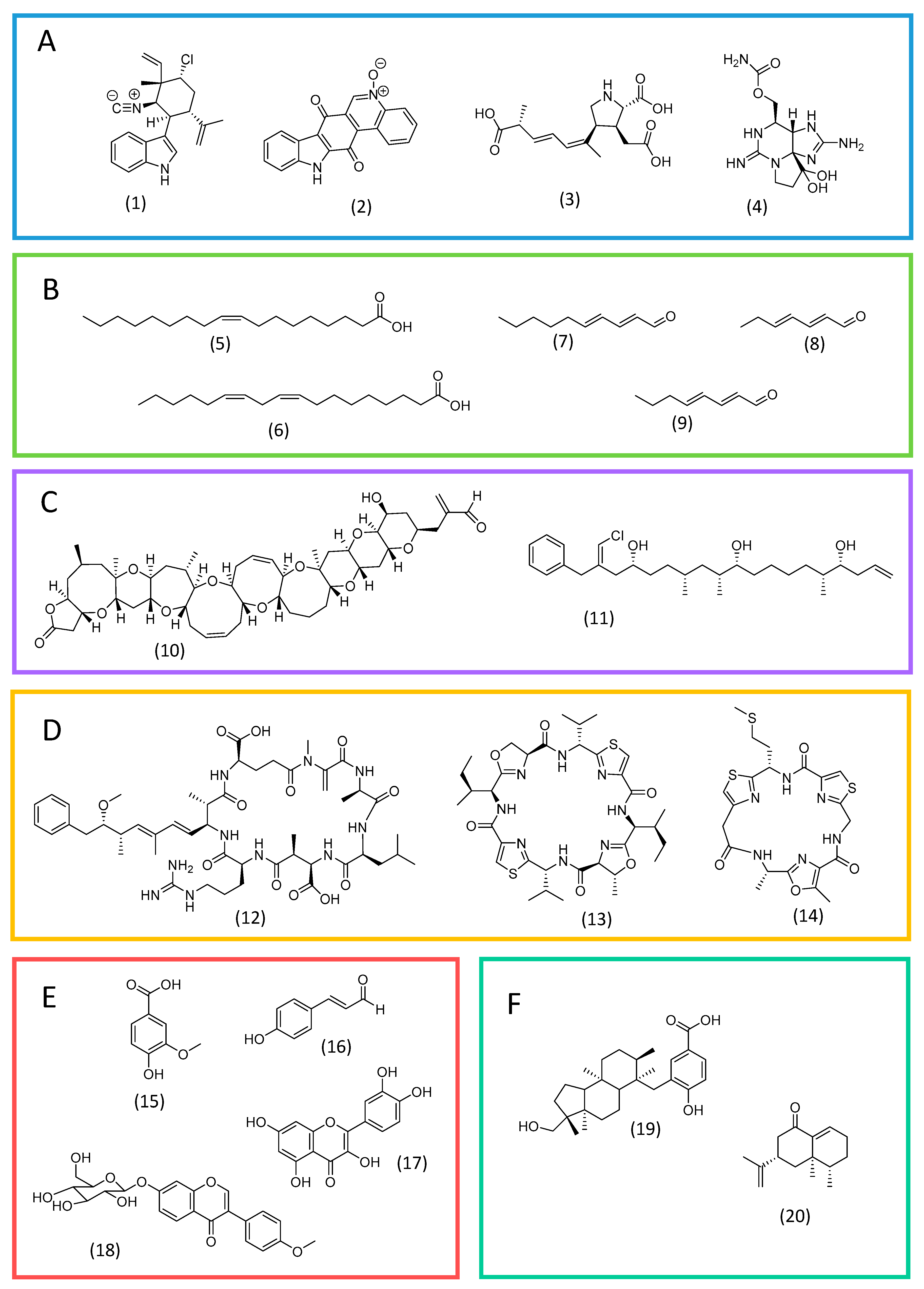
3.2. Fatty acids and Derivatives
Extracellular free fatty acids from microalgae have a possible allelopathic effect, controlling the growth of other microalgae and harmful organisms. Green microalgae, such as Chlorella sp. and Botryococcus braunii, are reported to secrete mixtures of common fatty acids (e.g., oleic and linoleic acids; Figure 2B) under certain conditions, which may favor their dominance [49,50][46][47]. Additionally, unsaturated fatty acids are reported to possess antimicrobial activity [51][48]. Substances derived from fatty acids can also act as allelochemicals. Polyunsaturated fatty acids (PUFAs), after various oxidative transformations, originate polyunsaturated aldehydes (PUAS) (e.g., 2E,4E-decadienal, 2E,4E-heptadienal, and 2E,4E-octadienal; Figure 2B), which play a role in communication and chemical defense in diatoms [11,39,52][11][36][49]. They exert a deleterious effect on grazers and competing microalgal species, and stimulate organic-matter-recycling bacteria [52][49].3.3. Polyketides
Polyketides are a large group of structurally diverse substances biosynthesized from carboxylic acid precursors through repeated cycles of condensation, reduction and dehydration reactions catalyzed by multifunctional enzymatic complexes called polyketide synthases (PKS). These substances are found mainly in dinoflagellates and cyanobacteria and are frequently associated with harmful blooms [39,53][36][50]. Examples include maitotoxin from the dinoflagellate Gambierdiscus sp., the largest and most toxic known polyketide [54,55][51][52], and also brevetoxins from Karenia brevis (e.g., brevetoxin A; Figure 2C; 10), a dinoflagellate associated with “red tides” [56][53]. Another remarkable example are trichophycins (e.g., trichophycin A; Figure 2C; 11), linear chlorinated polyketides from cyanobacteria from the genus Trichodesmium, which produce frequent blooms in the Pacific Ocean [57,58][54][55].3.4. Peptides
A significant amount of cyanobacterial allelochemicals are peptides. Most of them belong to the group of non-ribosomal peptides, which are biosynthesized by multifunctional enzyme complexes, the non-ribosomal peptide synthases (NRPS). NPRS are similar to PKS and assemble sequentially common amino acids but also unusual amino acids [12,39,59][12][36][56]. For example, microcystins are cyclic non-ribosomal peptides produced by cyanobacteria of the genus Anabaena, Microcystis, Planktothrix, Oscillatoria, and others. They are toxic for microalgae, aquatic plants, and for mammals and birds as well [12,60][12][57]. Microcystin-LR (Figure 2D; 12) is the most frequent of them [60][57]. Ribosomal peptides also occur in cyanobacteria. For instance, cyanobactins are small cyclic peptides formed solely from proteinogenic amino acids and reported in many genus, such as Anabaena, Lyngbya, Microcystis, Nostoc, and Trichodesmium [59,61][56][58]. They possess antimicrobial activity and probably have the function of inhibiting the growth of competing and harmful microorganisms. Patellamide A, from Prochloron, and tenuecyclamide C, from Trichodesmium erythraeum, are representatives of the group (Figure 2D; 13 and 14) [61][58].3.5. Phenolic Compounds
Phenolic compounds represent a large group of secondary metabolites, which play multiple roles in photosynthetic organisms, including in defense strategies and in communication with interacting species [62,63][59][60]. Phenolic substances are biosynthesized mainly by the phenylpropanoid pathway, having phenylalanine or tyrosine as precursors. In microalgae, knowledge about their biosynthetic pathways is still scarce, however, a recent study has shown that the enzymatic apparatus for key phenolic biosynthetic intermediates is well conserved in all major algal divisions, although the ability to biosynthesize some subclasses of phenolics seems to be restricted to some groups [62][59]. For instance, phenolic acids such as vanillic, ferulic, and coumaric acids are widespread. Flavonoids are frequent in green algae (e.g., quercetin and apigenin) and cyanobacteria, in which unusual structures have been reported (e.g., ononin from Synechocystis) (Figure 2E) [62][59].3.6. Terpenoids
Terpenoids are a prominent family of organic substances biosynthesized from C5 precursors, which are present in all living organisms [64][61]. Substances belonging to this group can also act as allelochemicals. Examples are comnostins, extracellular antibacterial diterpenoids produced by cyanobacteria Nostoc sp. [65][62], and the sesquiterpene eremophilone (Figure 2F), produced by cyanobacteria Calothrix sp. and toxic to invertebrates [66][63].3.7. Volatile Organic Compounds
A specific group of allelochemicals is the volatile organic compounds (VOCs). VOCs are a broad group of compounds that encompass substances from different chemical classes, ranging from small molecules such as methane, to terpenoids and compounds derived from PUFAs including hydrocarbons, aldehydes, and ketones [25][22]. With a complex classification, the World Health Organization (WHO) defines VOCs as having boiling point ranges that have to fall between 50 °C and 100 °C up to 240–260 °C [67][64]. VOCs produced by microalgae have been widely demonstrated to have different ecological functions, including allelopathy. Environmental factors such as light, temperature, nutrition, and abiotic stresses affect VOCs emission [68][65]. Functions such as defense mechanisms, stress response, intra- and inter-species communication, modulating predator-prey interaction, and affecting biochemistry, metabolism, and physiology processes are described. These emissions from microalgae occur in vivo, in cells undergoing senescence or apoptosis, or when they are perishing under predator attack [69,70][66][67]. The role and the possible impact that these compounds have has been extensively studied and, in the future, they could be exploited for industrial purposes [67][64].References
- Molisch, H. Der Einfluss einer Pflanze auf die Andere, Allelopathie. Nature 1938, 141, 493.
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592.
- Gomes, M.P.; Garcia, Q.S.; Barreto, L.C.; Pimenta, L.P.S.; Matheus, M.T.; Figueredo, C.C. Allelopathy: An overview from micro- to macroscopic organisms, from cells to environments, and the perspectives in a climate-changing world. Biologia 2017, 72, 113–129.
- Inderjit; Dakshini, K.M.M. Algal allelopathy. Bot. Rev. 1994, 60, 182–196.
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae Taxonomy and Breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53.
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232.
- Gallardo-Rodríguez, J.; Sánchez-Mirón, A.; García-Camacho, F.; López-Rosales, L.; Chisti, Y.; Molina-Grima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684.
- Stevens, P.F. Angiosperm Phylogeny and Diversification. In Encyclopedia of Evolutionary Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 78–83. ISBN 978-0-12-800426-5.
- Watson, S.B.; Whitton, B.A.; Higgins, S.N.; Paerl, H.W.; Brooks, B.W.; Wehr, J.D. Harmful Algal Blooms. In Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 873–920. ISBN 978-0-12-385876-4.
- Mathieu, A. Advances in Bioengineering Study of Microalgae. J. Pet. Environ. Biotechnol. 2022, 13, 1000453.
- Mendes, L.B.B.; Vermelho, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 152.
- Leflaive, J.; Ten-Hage, L. Algal and cyanobacterial secondary metabolites in freshwaters: A comparison of allelopathic compounds and toxins. Freshw. Biol. 2007, 52, 199–214.
- Shahbaz, A.; Hussain, N.; Saba, S.; Bilal, M. Actinomycetes, Cyanobacteria, and Fungi: A Rich Source of Bioactive Molecules. In Microbial Biomolecules; Elsevier: Amsterdam, The Netherlands, 2023; pp. 113–133. ISBN 978-0-323-99476-7.
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L. Isolation, Structure Determination, and Biological Activity of Lyngbyabellin A from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 611–615.
- Legrand, C.; Rengefors, K.; Fistarol, G.O.; Granéli, E. Allelopathy in phytoplankton—Biochemical, ecological and evolutionary aspects. Phycologia 2003, 42, 406–419.
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021, 54, 102213.
- Molisch, H.; Narwal, S.S. The Influence of One Plant on Another, Allelopathy; Published by Scientific Publishers (India) for International Allelopathy Foundation, Hisar: Jodhpur, India, 2001; ISBN 978-81-7233-285-3.
- Caruana, A.M.N.; Amzil, Z. Microalgae and Toxins. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 263–305. ISBN 978-0-12-811405-6.
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447.
- Śliwińska-Wilczewska, S.; Wiśniewska, K.; Konarzewska, Z.; Cieszyńska, A.; Felpeto, A.B.; Lewandowska, A.U.; Latała, A. The current state of knowledge on taxonomy, modulating factors, ecological roles, and mode of action of phytoplankton allelochemicals. Sci. Total. Environ. 2021, 773, 145681.
- Wang, Z.; Wang, C.; Li, W.; Wang, M.; Xiao, L. Interspecies competition between Scrippsiella acuminata and three marine diatoms: Growth inhibition and allelopathic effects. Aquat. Toxicol. 2021, 237, 105878.
- Kumari, P.; Cna’ani, A.; Didi-Cohen, S.; Tzin, V.; Khozin-Goldberg, I. Nitrogen Deprivation-Induced Production of Volatile Organic Compounds in the Arachidonic-Acid-Accumulating Microalga Lobosphaera incisa Underpins Their Role as ROS Scavengers and Chemical Messengers. Front. Mar. Sci. 2020, 7, 410.
- Sartori, R.B.; Siqueira, S.F.; Maroneze, M.M.; Fagundes, M.B.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgal secondary metabolites: Effect of climatic variables, seasons, and photocycles on the biogeneration of volatile organic compounds (VOCs). J. Appl. Phycol. 2021, 33, 1457–1472.
- Grabski, K.; Tukaj, Z. Autoinduction activity of a conditioned medium obtained from high density cultures of the green alga Scenedesmus subspicatus. J. Appl. Phycol. 2007, 20, 323–330.
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.-S.; Zimba, P.V.; do Carmo Bittencourt-Oliveira, M.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77.
- Ali, S.S.; Mastropetros, S.G.; Schagerl, M.; Sakarika, M.; Elsamahy, T.; El-Sheekh, M.; Sun, J.; Kornaros, M. Recent advances in wastewater microalgae-based biofuels production: A state-of-the-art review. Energy Rep. 2022, 8, 13253–13280.
- Casanova, L.M.; Mendes, L.B.B.; Corrêa, T.D.S.; da Silva, R.B.; Joao, R.R.; Macrae, A.; Vermelho, A.B. Development of Microalgae Biodiesel: Current Status and Perspectives. Microorganisms 2022, 11, 34.
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manag. 2022, 320, 115897.
- Loftus, S.E.; Johnson, Z.I. Cross-study analysis of factors affecting algae cultivation in recycled medium for biofuel production. Algal Res. 2017, 24, 154–166.
- Lu, Z.; Loftus, S.; Sha, J.; Wang, W.; Park, M.S.; Zhang, X.; Johnson, Z.I.; Hu, Q. Water reuse for sustainable microalgae cultivation: Current knowledge and future directions. Resour. Conserv. Recycl. 2020, 161, 104975.
- Thomas, P.K.; Hietala, D.C.; Cardinale, B.J. Tolerance to allelopathic inhibition by free fatty acids in five biofuel candidate microalgae strains. Bioresour. Technol. Rep. 2023, 21, 101321.
- Qixin, L.; Xuan, F.; Zhiya, S.; Wenxin, S.; Shuo, W.; Ji, L. Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour. Technol. 2022, 354, 127161.
- Satake, M.; Honma, D.; Watanabe, R.; Oshima, Y. Alexandrolide, a diatom growth inhibitor isolated from the dinoflagellate Alexandrium catenella. Tetrahedron Lett. 2019, 60, 1341–1344.
- Pouvreau, J.-B.; Housson, E.; Le Tallec, L.; Morançais, M.; Rincé, Y.; Fleurence, J.; Pondaven, P. Growth inhibition of several marine diatom species induced by the shading effect and allelopathic activity of marennine, a blue-green polyphenolic pigment of the diatom Haslea ostrearia (Gaillon/Bory) Simonsen. J. Exp. Mar. Biol. Ecol. 2007, 352, 212–225.
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754.
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the post-genomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785.
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2020, 38, 103–129.
- Doan, N.T.; Rickards, R.W.; Rothschild, J.M.; Smith, G.D. Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J. Appl. Phycol. 2000, 12, 409–416.
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358.
- Quilliam, M.A.; Wright, J.L.C. The Amnesic Shellfish Poisoning Mystery. Anal. Chem. 1989, 61, 1053A–1060A.
- Takemoto, T.; Daigo, K. Constituents of Chondria Armata. Chem. Pharm. Bull. 1958, 6, 578–580.
- Orr, R.J.S.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolution and Distribution of Saxitoxin Biosynthesis in Dinoflagellates. Mar. Drugs 2013, 11, 2814–2828.
- Murray, S.A.; Mihali, T.K.; Neilan, B.A. Extraordinary Conservation, Gene Loss, and Positive Selection in the Evolution of an Ancient Neurotoxin. Mol. Biol. Evol. 2011, 28, 1173–1182.
- Schantz, E.J.; Lynch, J.M.; Vayvada, G.; Matsumoto, K.; Rapoport, H. The Purification and Characterization of the Poison Produced by Gonyaulax catenella in Axenic Culture. Biochemistry 1966, 5, 1191–1195.
- Jackim, E.; Gentile, J. Toxins of a Blue-Green Alga: Similarity to Saxitoxin. Science 1968, 162, 915–916.
- Chiang, I.-Z.; Huang, W.-Y.; Wu, J.-T. Allelochemicals of Botryococcus braunii (Chloroficeae). J. Phycol. 2004, 40, 474–480.
- DellaGreca, M.; Zarrelli, A.; Fergola, P.; Cerasuolo, M.; Pollio, A.; Pinto, G. Fatty Acids Released by Chlorella vulgaris and Their Role in Interference with Pseudokirchneriella subcapitata: Experiments and Modelling. J. Chem. Ecol. 2010, 36, 339–349.
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44.
- Cózar, A.; Morillo-García, S.; Ortega, M.J.; Li, Q.P.; Bartual, A. Macroecological patterns of the phytoplankton production of polyunsaturated aldehydes. Sci. Rep. 2018, 8, 12282.
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320.
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some Chemical Properties of Maitotoxin, a Putative Calcium Channel Agonist Isolated from a Marine Dinoflagellate. J. Biochem. 1988, 104, 184–187.
- Reyes, J.G.; Sánchez-Cárdenas, C.; Acevedo-Castillo, W.; Leyton, P.; López-González, I.; Felix, R.; Gandini, M.A.; Treviño, M.B.; Treviño, C.L. Maitotoxin: An Enigmatic Toxic Molecule with Useful Applications in the Biomedical Sciences. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 677–694. ISBN 978-0-429-09621-1.
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health Perspect. 2005, 113, 621–625.
- McManus, K.M.; Kirk, R.D.; Via, C.W.; Lotti, J.S.; Roduit, A.F.; Teta, R.; Scarpato, S.; Mangoni, A.; Bertin, M.J. Isolation of Isotrichophycin C and Trichophycins G–I from a Collection of Trichodesmium thiebautii. J. Nat. Prod. 2020, 83, 2664–2671.
- Bertin, M.J.; Wahome, P.G.; Zimba, P.V.; He, H.; Moeller, P.D.R. Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom. Mar. Drugs 2017, 15, 10.
- Micallef, M.L.; D’Agostino, P.M.; Al-Sinawi, B.; Neilan, B.A.; Moffitt, M.C. Exploring cyanobacterial genomes for natural product biosynthesis pathways. Mar. Genom. 2014, 21, 1–12.
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249.
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225.
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171.
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913.
- Oldfield, E.; Lin, F.-Y. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. 2011, 51, 1124–1137.
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel Extracellular Diterpenoids with Biological Activity from the Cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343.
- Höckelmann, C.; Becher, P.G.; von Reuß, S.H.; Jüttner, F. Sesquiterpenes of the Geosmin-Producing Cyanobacterium Calothrix PCC 7507 and their Toxicity to Invertebrates. Z. Naturforsch C. J. Biosci. 2009, 64, 49–55.
- Pozzer, A.C.; Gómez, P.A.; Weiss, J. Volatile organic compounds in aquatic ecosystems—Detection, origin, significance and applications. Sci. Total. Environ. 2022, 838, 156155.
- Zuo, Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019, 10, 491.
- Achyuthan, K.E.; Harper, J.C.; Manginell, R.P.; Moorman, M.W. Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity? Metabolites 2017, 7, 39.
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants. Horticulturae 2021, 7, 278.
