Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Irene Dini.
Seaweeds or algae are marine autotrophic organisms. They produce nutrients (e.g., proteins, carbohydrates, etc.) essential for the survival of living organisms as they participate in biochemical processes and non-nutritive molecules (such as dietary fibers and secondary metabolites), which can improve their physiological functions. Seaweed polysaccharides, fatty acids, peptides, terpenoids, pigments, and polyphenols have biological properties that can be used to develop food supplements and nutricosmetic products as they can act as antibacterial, antiviral, antioxidant, and anti-inflammatory compounds.
- food supplement
- nutraceutical
- seaweed
- circular economy
- biodiversity recycling
- eco-friendly product
1. Introduction
The main goal of the Circular Economy is to reuse and recycle natural resources to minimize health, energy, and environmental impacts. The European citizen produces around 5 tonnes of waste, much of which finishes in incinerators or landfills, and a little is recycled [1]. Waste management policies have been investigated to avoid landfills and allow the recovery of renewable energy and recycled materials [2]. Organizations have developed circular waste management systems, promoting resource flow and enhancing product sustainability and processes [3]. Consumption of eco-friendly products and decreasing waste are crucial to achieving the European sustainable goals. Ten megatrends were recognized for 2022 by New Nutrition Business for food, nutrition, and health. Sustainability came fifth [4]. Representative population surveys indicate that many people (amongst them young consumers) wish to contribute to sustainable development [5,6,7,8,9,10][5][6][7][8][9][10]. Buying eco-friendly products is considered one way to intervene. In the European Union, 26% of consumers purchase eco-friendly products, and 54% rarely use such items [11]. The global market value of natural and organic skincare products will probably grow from 9.9 billion dollars in 2021 to 20.4 billion dollars by 2030 [12]. The organic segment (made from plant ingredients that have been grown in soil free of fungicides, pesticides, synthetic fertilizers, and herbicides, and genetically modified organisms) was valued at $28,323.2 million in 2021 and is expected to reach $74,058.5 million by (CAGR of 9.8%) [13]. This data supports the significant contribution of the cosmetics market worldwide to environmental sustainability. The seaweed waste (e.g., beach-casts) [14] and invasive species valorization [15], which are of no commercial value and must be disposed of in landfills, could represent an eco-friendly, attractive low-cost source for supplements and cosmetics formulations. Some scientific studies have shown the potential skincare properties of algae bioactive metabolites [16,17,18,19][16][17][18][19]. In seaweeds are found compounds with low allergen and cytotoxic profiles [20], such as peptides, polysaccharides, fatty acids, vitamins, carotenoids, phlorotannins, tocopherols, phycobilins, phycocyanins, and sterols [21,22,23,24][21][22][23][24] that can act as antioxidants, photoprotective, moisturizing, anti-inflammatory, antiallergic, anti-acne, anti-wrinkling, antiaging, antimicrobial, and whitening bioactive compounds [25,26,27,28][25][26][27][28].
2. Nutricosmetic Revolution
The term “nutricosmetic” indicates the association of food supplements and cosmeceuticals to improve skin care. Nutricosmetic formulations optimize the intake of nutritional macro and micro elements to meet the demands of the skin and appendages, improving their conditions and delaying aging [29,30,31][29][30][31]. A food supplement is a consumer product that aims to supplement the regular diet. Products based on vitamins, minerals, antioxidants, and extracts of vegetable origin, single and multi-compound, in pre-dosed forms with nutritional power or biological effect, fall into the vast category of food supplements [32]. Cosmetics represent a highly heterogeneous category of daily-use consumer products. In the European Union, Regulation (EC) no. 1223/2009 in Article 2 defines “cosmetic product” as “any substance or mixture intended to be applied on the external surfaces of the human body (epidermis, hair system, and hair, nails, lips, external genital organs) or the teeth and on the mucous membranes of the mouth for the sole or primary purpose of cleaning them, perfuming them, modifying their appearance, protecting them, keeping them in good condition or correcting body odors” [33]. A substance or mixture intended to be ingested, inhaled, injected, or implanted in the human body is not considered a cosmetic product. Nutricosmetic formulations combine the two previous formulations’ beneficial effects through an integrated “in and out” approach.3. Algae (Seaweeds)
Algae are a group of photosynthetic organisms that differ in structure and size. They can grow in freshwater, marine water, deep oceans, and rocky shores. The bionetwork comprises 36,000 different kinds of algae. The seaweed macroalgae are multicellular organisms rich in lipids and proteins (40% and 71% of their dry weight) that can measure from a few centimeters to a meter, while the microalgae are microscopic unicellular carbohydrate-rich organisms [34]. Macroalgae are grouped in Chlorophyta (green algae), Phaeophyta (brown algae), and Rhodophyta (red algae) according to their pigment and chlorophyll profile (Figure 1).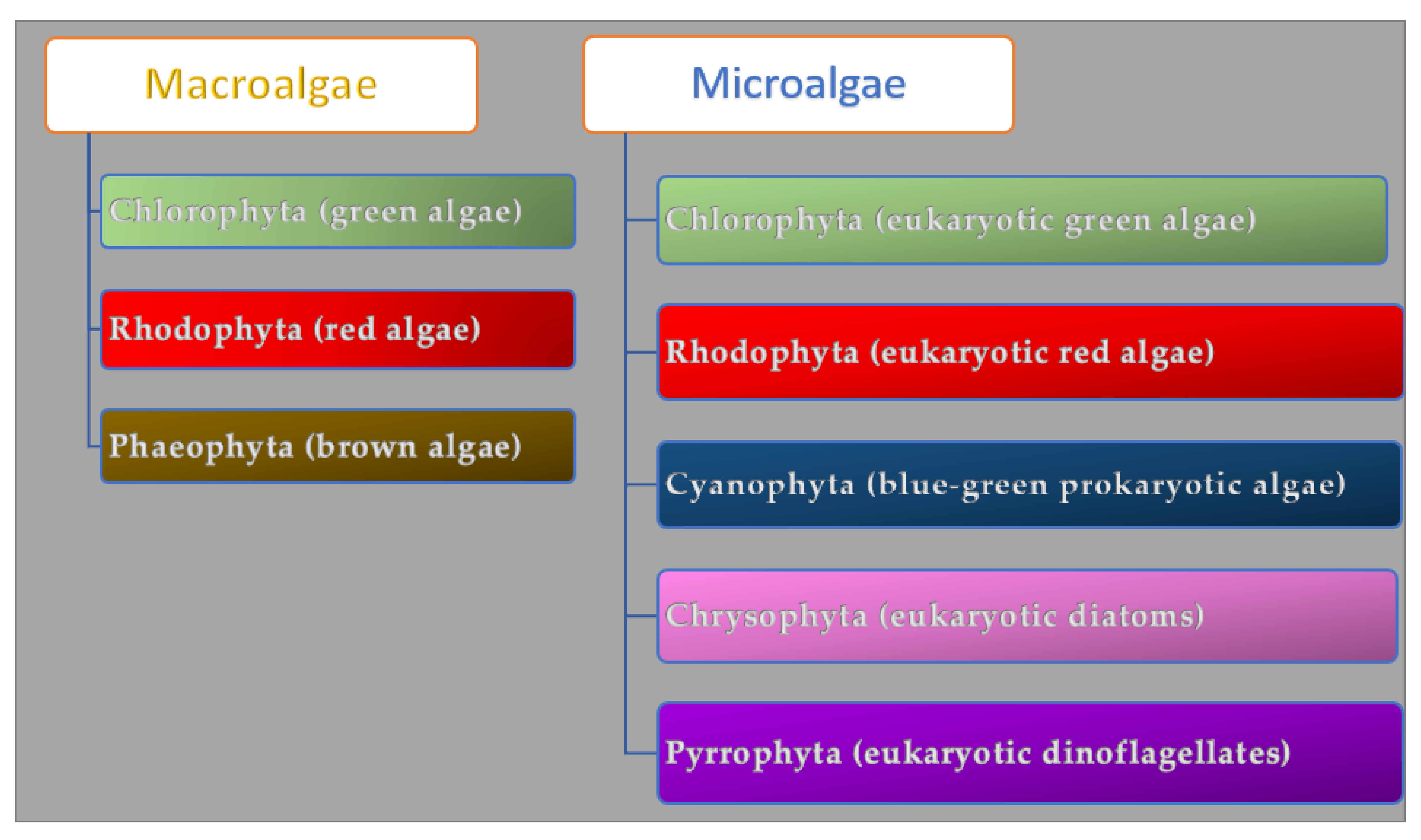
Figure 1. Algae classification.
4. Algae Metabolites
4.1. Polysaccharides
Marine macroalgae are good carbohydrate sources (mainly polysaccharides and low concentration of disaccharides and monosaccharides) whose content is from 5 to 75% (w/w, DW) based on the age, period, species, and harvesting site [52,53][52][53]. Polysaccharides in seaweeds can be sulfated and non-sulfated [54]. They constitute the algae cell walls and are species-specific (Figure 2) [55,56][55][56]. They have some technological, rheological, and biological activities. They can have a prebiotic effect and improve gut human microbiota performance [57].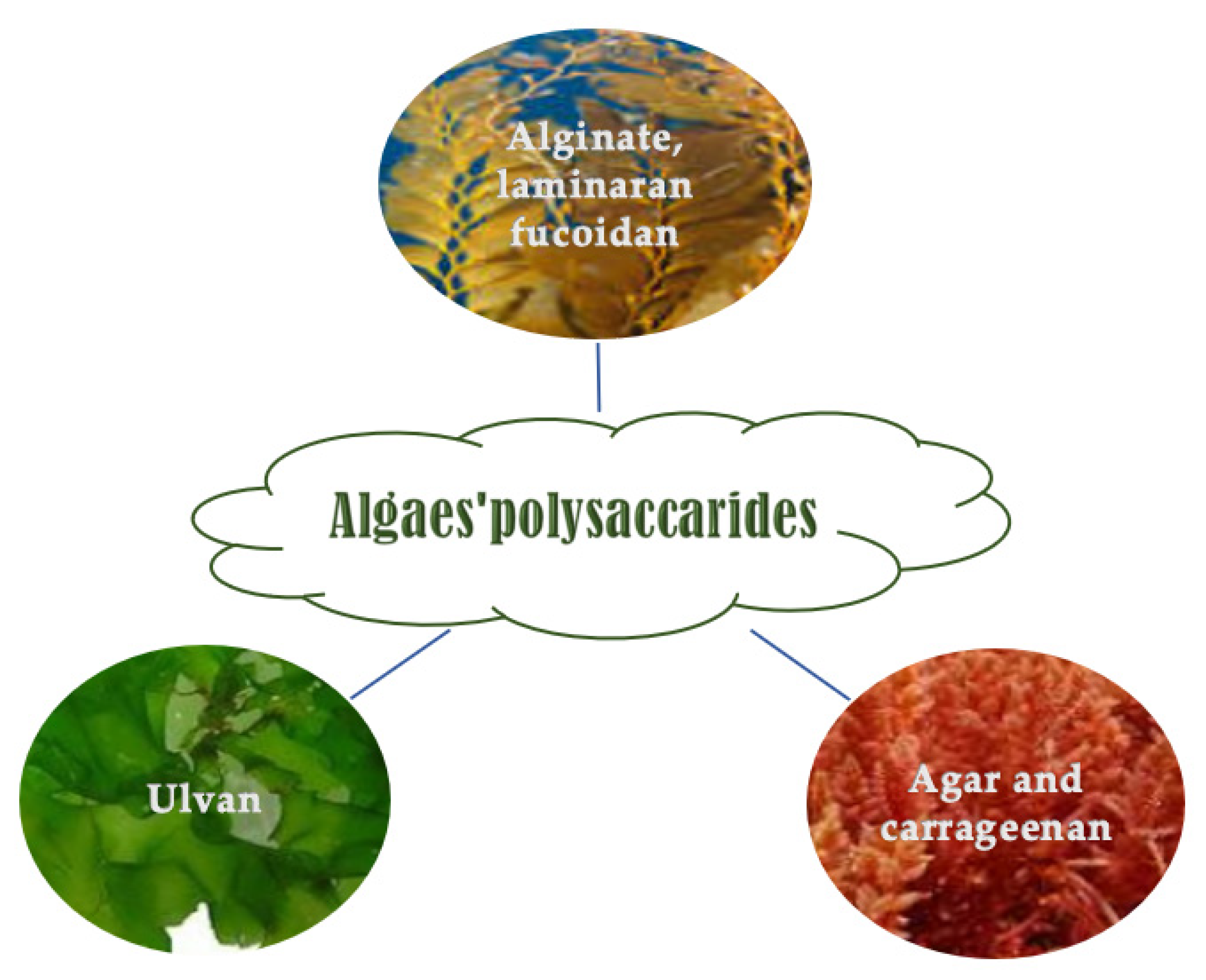
Figure 2. Polysaccharides occurrence in the function of algae class.
4.1.1. Brown Algae Polysaccharides
Brown macroalgae are composed of sulfated and branched α-l-fucans containing predominantly sulfated l-Fucp (<90%), other monosaccharides (e.g., d-Manp, d-Galp, and d-Xylp), and uronic acids (d-GlcAp and d-GalAp). Brown algae polysaccharides have antioxidant, antiinflammatory, and antibacterial activity against E. coli, S. epidermidis, S. aureus, and B. licheniformis [58,59][58][59]. Ascophyllans (xylofucoglucuronanes) have a poly-(1→4)-β-d-glucuronan skeleton linked to l-Fucp and d-Xylp sulfated in position C-4 [60]. Sargassans (glucuronofucogalactans), identified in the genus Sargassum (e.g., Sargassum linifolium), have a poly-(1→4)-β-d-glucuronan skeleton linked with d-Manp residues [61]. Fucoidans have low shear-thinning performance and low viscoelastic physical characteristics (they are affected by monovalent and divalent salts) [62]. They are biocompatible, non-toxic, biodegradable [63[63][64],64], and have antioxidant and antiradical properties [65,66,67,68][65][66][67][68]. Fucoidans can promote skin firmness, elasticity, brightness, hair growth, safety, cleanliness, rigidity, and gloss [69]. They prevent and treat skin photoaging, decreasing wrinkle-related enzymes (e.g., collagenase, gelatinase, elastase) [70[70][71][72],71,72], improving collagen synthesis [73], controlling matrix metalloproteinases and avoiding the extracellular matrix’s ruin [74,75,76,77][74][75][76][77]. Laminarins (also laminarans), identified in laminaria present in the North Atlantic, have a degree of polymerization of 15–40 and molecular weight (Mw of 2–10 kDa). They are β-(1→3)-d-glucans. The laminaribiosis are the diholosidic repeating unit consisting of β-(1→6)-d-Glcp [78]. Laminarins are biocompatible, have low cell toxicity, are biodegradable, and show some bioactivity, such as anti-inflammatory, antioxidant [79] anti-photoaging and regenerative abilities [80]. Alginate(s) are polysaccharides composed of α-l-guluronic acid (l-GulpA) (1C4 ring conformation) and (1→4)-β-d-mannuronic acid (d-ManpA) (4C1 ring conformation) [81] arranged in both homogeneous and heterogeneous blocks [81]. Alginates are used in the food, feed, cosmetic, and drug industries as gelifying and thickening agents, and bioactive molecules against allergy [82] and obesity [83,84][83][84].4.1.2. Red Algae Polysaccharides
Red algae (Rhodophyta) contain water-soluble sulfated galactan (e.g., agarocolloids and carrageenans), constructed based on (1→4)-α-Galp and (1→3)-β-Galp units [53]. Carrageenans have gel and texture properties. They are the fourth principal hydrocolloids used by the food industry, after starch, gelatin, and pectin [85]. Sulfated dioside are linear polymers of carrabiosis that can contain 4-α-d-Galp and 3-β-d- Galp, other monosaccharides (Xylp, GlcAp, Glcp, and GalAp), methyl ether groups, and pyruvic acid ketals. They are extracted from Agardhiella, Eucheuma, Chondrus, Gigartina, Furcellaria, and Hypnea [53,86][53][86]. Agarans are sulfated galactan containing 4-α-l-Galp [87]. Agarans based on the percentages of 3-6-α-l-AnGalp residues and sulfate groups are defined agaroids that are weak gelling molecules (divided into funorans and porphyrans), and agars (high gelling molecules). Agaroids are extracted from Porphyra species, e.g., P. capensis, Porphyra haitanensis [88], or P. umbilicalis [89]. Agar has cosmetic and pharmaceutical applications as a thickener agent and an ingredient to carry and release drugs in capsules and tablets [89,90][89][90].4.1.3. Green Seaweed Polysaccharides
Chlorophyceae contain sulfated polyholosides [91]. Polyholosides are distinct in sulfated xylorhamnoglycuronans, called ulvans [92,93,94,95][92][93][94][95], sulfated arabinoxylogalactans or xyloarabinogalactans (composed of Araf, d-Galp, l- and d-Xylp units) present in the orders of Cladophorales and Bryopsidales, and sulfated rhamnogalactogalacturonanes or glucuronoxylorhamnogalactans extracted from Ulvales [96]. Ulvans are used as gelling [97] and antiaging agents [98].4.2. Lipids
Algae contain omega-3 and omega-6 polyunsaturated fatty acids (PUFA; usually under 5%). The γ-linolenic acid, eicosapentaenoic acid, arachidonic acid, and docosahexaenoic acid are the most abundant. Phaeophyta algae have a C18-PUFAs profile next to green algae and a C20-PUFAs profile identical to red algae. Chlorophyta species have higher levels of C18-PUFAs than C20-PUFAs. In Rhodophyta happen the contrary. Green algae contain higher DHA (docosahexaenoic acid) levels (e.g., Chlorophyta algae genus Tetraselmis). Finally, red and brown algae have predominantly EPA (eicosapentaenoic acid), arachidonic acid [99[99][100],100], and phospholipids [101,102,103,104,105][101][102][103][104][105]. Polyunsaturated fatty acids can improve skin barrier protection [106,107][106][107] and regulate inflammatory responses [108]. Lipids in cosmetic formulations can act as moisturizing agents (forming a waterproof film on the skin to avoid water evaporation from the surface) [109], emollient [110], and softening agents (they make the corneocyte’s edges smoother) [36], surfactants [111], and emulsifiers (they decrease the surface tension) [112], texturizers (they improve the spreadability of gel-like products), and as color and fragrance carriers [113].4.3. Proteins and Derivatives
Seaweeds are a rich source of proteins (in single or conjugate form) and protein derivatives (e.g., free amino acids and peptides) [23]. Red algae have the highest proteins and derivative contents (up to 47%), green algae have medium levels (between 9–26%), and brown algae contain the lowest concentrations (3–15%) [114]. Protein and bioactive peptides have high antioxidant, anti-inflammatory, skin proactive, and antiaging properties [115,116,117][115][116][117]. Pedoclimatic conditions affect the proteins, peptides, and amino acids contents in algae. Taurine extracted from the thalli of Euthora cristata, Ahnfeltia plicata, and Ceramium virgatum has antioxidant and chelating abilities [118,119][118][119]. The peptides (PYP1-5, and Porphyra 334) from Porphyra yezoensis f. coreana increase collagen and elastin levels and reduce the expression of matrix metalloproteinases (MMP) MMP-1 and MMP-8 [120]. Mycosporine-like amino acids (MAAs) (Figure 3) are secondary metabolites with low molecular weight (<400 Da) synthesized for protection against solar radiation and found in the cell cytoplasm [121]. Mycosporine-like amino acids are made by cycloheximide or cyclohexenone conjugated to amino acid or an imino alcohol residue [122]. They are extracted mainly from Rhodophyceae (e.g., shinorine, asterina, porphyra, palythine, polyphenol, mycosporine-glycine, and palythene) [123,124][123][124] and from Asparagopsis armata, Mastocarpus stellatus, Chondrus crispus, Gelidium sp., Palmaria palmata, Gracilaria cornea, Grateloupia lanceola, Solieria chordalis, and Curdiea racovitzae. This compound class has shown antioxidant, photoprotective, anti-proliferative [125], anti-aging, and anti-inflammatory activities [126].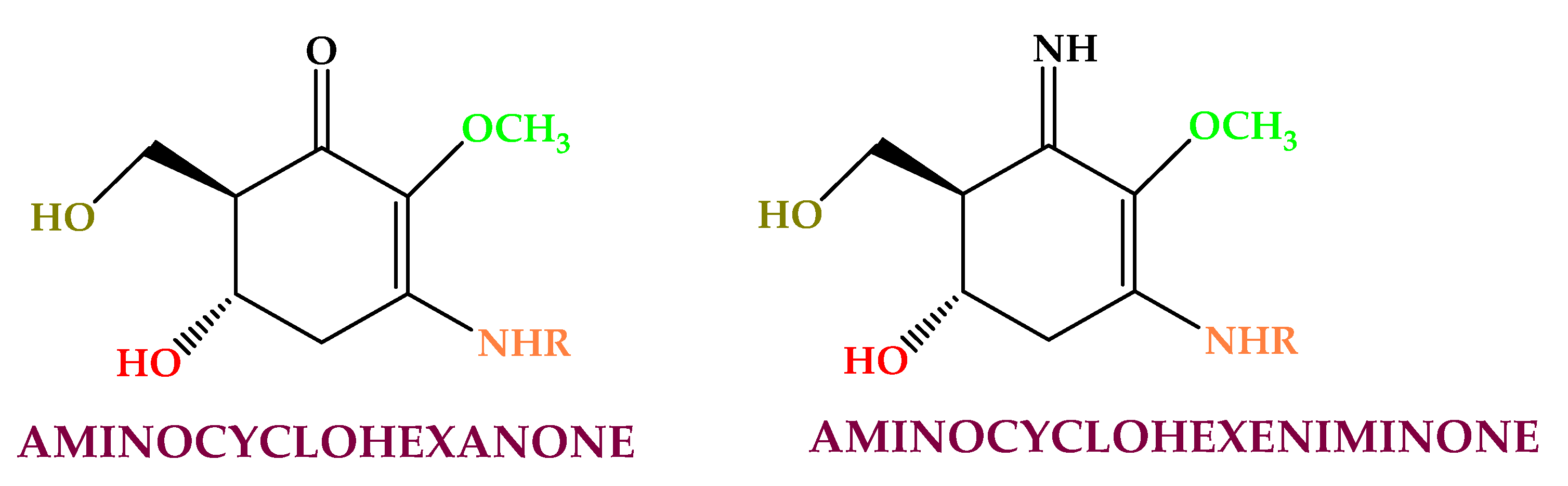
Figure 3. Algaes’ mycosporine-like amino acids found in algae.
4.4. Phenolics
Phenolic compounds are secondary plant metabolites with one or more aromatic rings with one or more -OH phenolic groups (e.g., phlorotannins, bromophenols, flavonoids, phenolic terpenoid, and mycosporine-like amino acids) [130]. They can defend algae from pedoclimatic injuries and parasite attacks [131,132][131][132]. The biological activities attributed to the algae’s phenolic compounds are summarized in Figure 4 [133].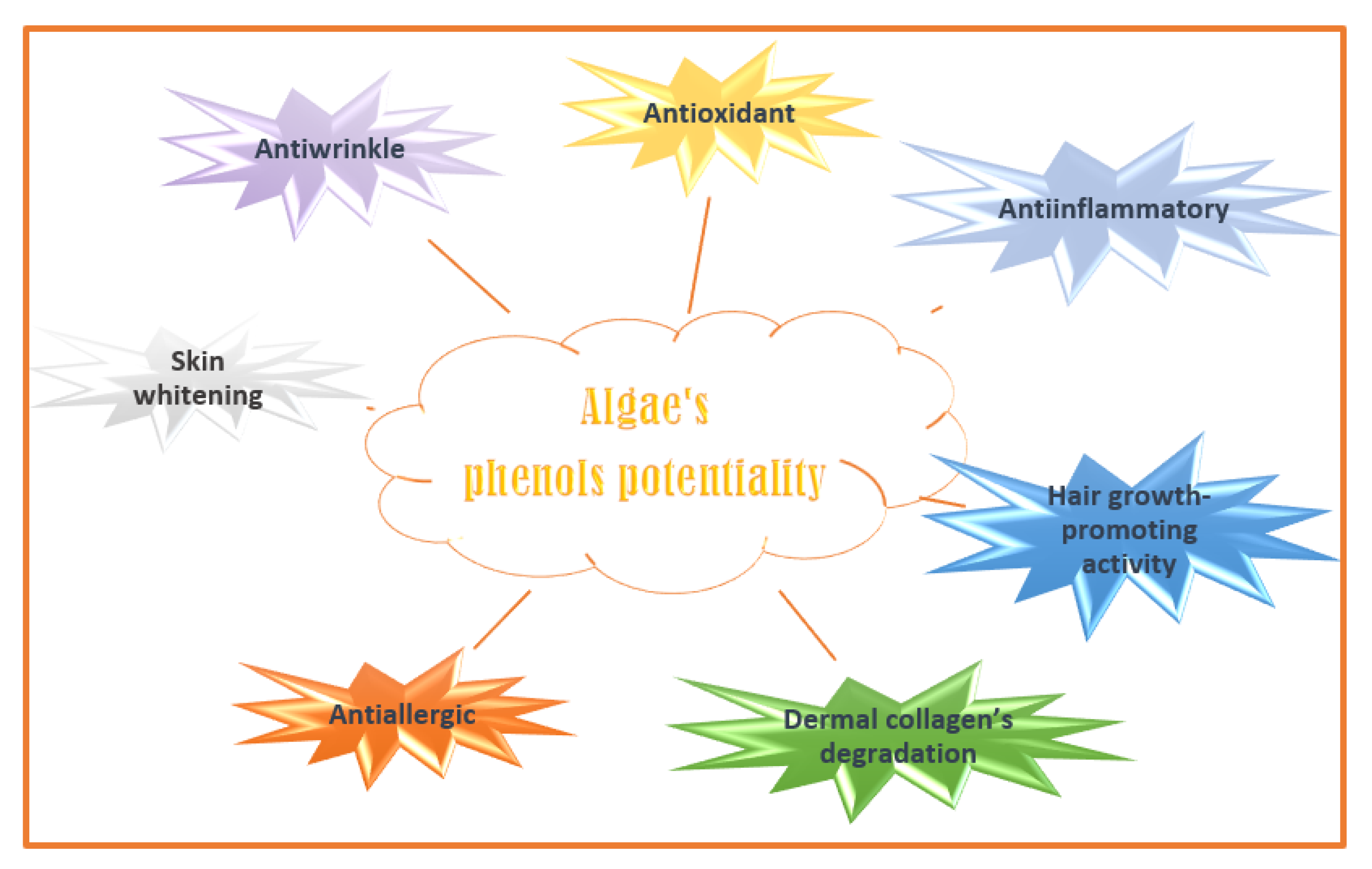
Figure 4. Algaes’ phenols potentialities in nutricosmetic formulation.
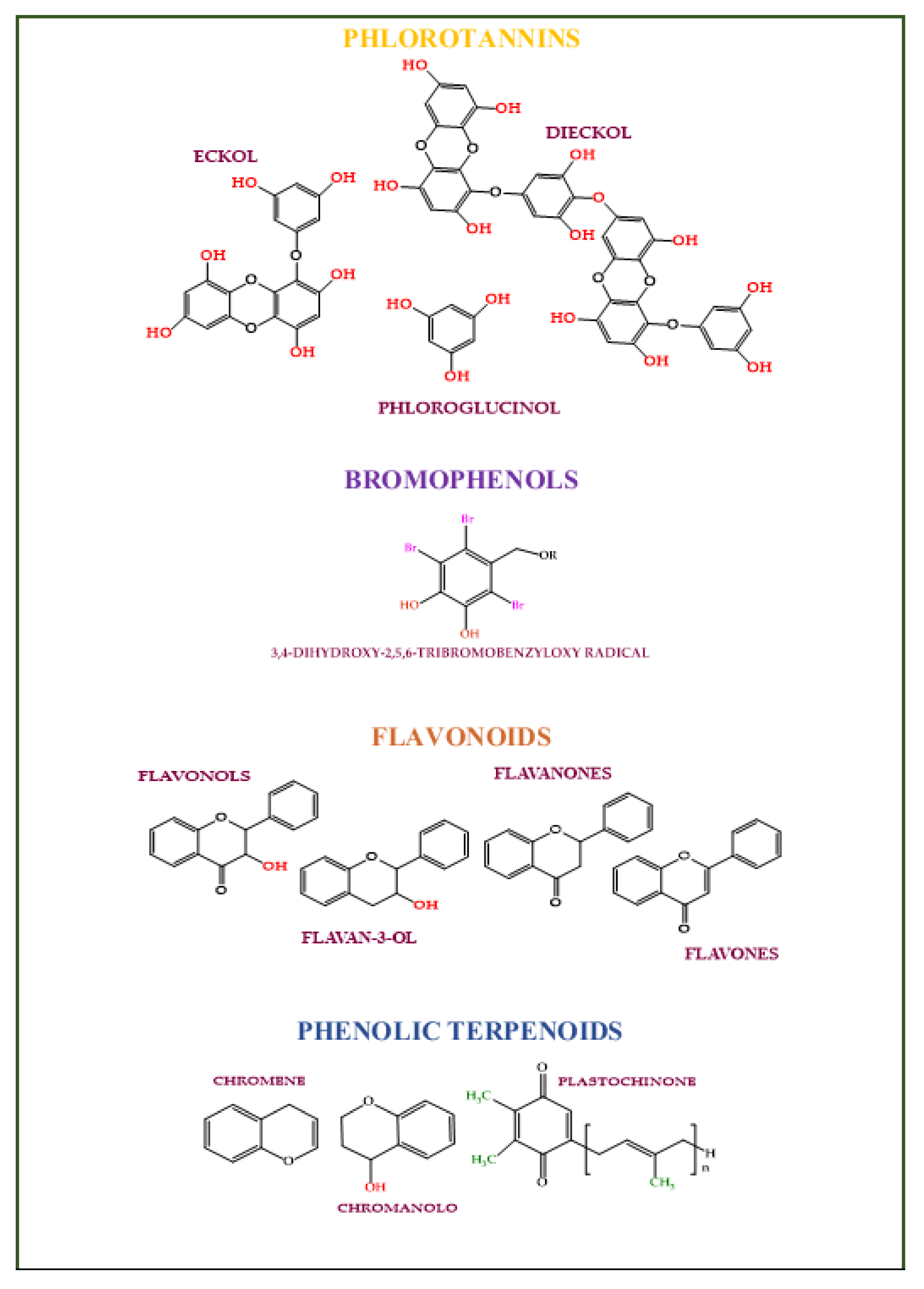
Figure 5. The main class of phenolic compounds found in algae.
References
- Eurostat Statistics Explained: Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 1 January 2023).
- Colasante, A.; D’Adamo, I.; Morone, P.; Rosa, P. Assessing the Circularity Performance in a European Cross-Country Comparison. Environ. Impact Assess. Rev. 2022, 93, 106730.
- Luo, Y.; Song, K.; Ding, X.; Wu, X. Environmental sustainability of textiles and apparel: A review of evaluation methods. Environ. Impact Assess. Rev. 2021, 86, 106497.
- New Nutrition. 10 Key Trends in Food, Nutrition & Health. 2022. Available online: New-nutrition.com (accessed on 22 July 2021).
- Eurostat (European Commission). Sustainable Development in the European Union: 2015 Monitoring Report of the E.U. Sustainable Development Strategy: 2015 Edition; Publications Office of European Union: Luxembourg, 2015.
- Eurostat (European Commission). Sustainable Development in the European Union: Monitoring Report on Progress Towards the S.D.G.s in an E.U. Context: 2017 Edition; Publications Office of European Union: Luxembourg, 2018.
- European Commission. Directorate-General for Communication. Towards a Sustainable Europe by 2030: Reflection Paper; Publications Office of European Union: Luxembourg, 2019.
- Cho, Y.-N.; Soster, R.L.; Burton, S. Enhancing Environmentally Conscious Consumption through Standardized Sustainability Information. J. Consum. Aff. 2017, 52, 393–414.
- Yarimoglu, E.; Binboga, G. Understanding sustainable consumption in an emerging country: The antecedents and consequences of the ecologically conscious consumer behavior model. Bus. Strat. Environ. 2018, 28, 642–651.
- Lendvai, B.M.; Kovács, I.; Lisányi, J.B. Helyi élelmiszer termékekkel kapcsolatos észlelései. Generation Z’s perceptions of local food products. In Proceedings of the Georgikon Conference, Keszthely, Hungary, 7 October 2021.
- Eurobarometer. Attitudes of Europeans towards Building the Single Market for Green Products. Flash Eurobarometer 367. 2013. Available online: http://ec.europa.eu/public_opinion/flash/fl_367_en.pdf (accessed on 22 January 2014).
- Statista. Value of the Natural and Organic Skin Care Products Market from 2021 to 2030 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/1116674/global-market-value-for-natural-organic-skin-care/ (accessed on 1 March 2021).
- Sustainable Personal Care Market by Nature (Organic, Natural and Green), by Type (Skin Care, Hair Care, Oral Care, Hygiene Products, Others), by Sales Channel (Hypermarkets and Supermarkets, Specialty Stores, Online Retail, Others): Global Opportunity Analysis and Industry Forecast, 2021–2031. Available online: https://www.alliedmarketresearch.com/sustainable-personal-care-market-A16262 (accessed on 1 May 2022).
- Zárate, R.; Portillo, E.; Teixidó, S.; de Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and cosmeceutical potential of Seaweed Beach-casts of Macaronesia. Appl. Sci. 2020, 10, 5831.
- Félix, R.; Carmona, A.M.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Industry-friendly hydroethanolic extraction protocols for Grateloupia turuturu UV-shielding and antioxidant compounds. Appl. Sci. 2020, 10, 5304.
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vazquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48.
- Wahyuni, T. The Potential and Application of Eucheuma sp. For Solid Soap: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 750, 012048.
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688.
- Lafarga, T.; Acién-Fernándeza, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal. Res. 2020, 48, 101909.
- Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Structures and Antiallergic Activities of Natural Products from Marine Organisms. Mar. Drugs 2023, 21, 152.
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464.
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780.
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68.
- Kharkwal, H.; Joshi, D.; Panthari, P.; Pant, M.K.; Kharkwal, A.C. Algae as future drugs. Asian J. Pharm. Clin. Res. 2012, 5, 1–4.
- Senevirathne, W.S.M.; Kim, S.K. Cosmeceuticals from Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 694–713.
- Vo, T.; Ngo, D.; Kang, K.; Jung, W.; Kim, S. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138.
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182.
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV Photoprotection, Cytotoxicity and Immunology Capacity of Red Algae Extracts. Molecules 2019, 24, 341.
- Dini, I. Bio Discarded from Waste to Resource. Foods 2021, 10, 2652.
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytot. Res. 2019, 33, 3054–3063.
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921.
- Dini, I. The commercial importance to develop validated analytical methods to define phytochemical levels in herbal medicinal products. Phytot. Res. 2022, 36, 3675–3677.
- Regulation (E.C.) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 31 July 2022).
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941.
- Freitas, F.; Torres, C.A.V.; Araújo, D.; Farinha, I.; Pereira, J.R.; Concórdio-Reis, P.; Reis, M.A.M. Advanced Microbial Polysaccharides. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 19–62.
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Sci. Eng. Technol. 2018, 7, 1269–1278.
- Brindhadevi, K.; Mathimani, T.; Rene, E.R.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 284.
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145.
- del Río, P.G.; Gomes-Dias, J.S.; Rocha, C.M.R.; Romaní, A.; Garrote, G.; Domingues, L. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour. Technol. 2019, 299, 122613.
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501.
- Ashokkumar, V.; Jayashree, S.; Kumar, G.; Sharmili, S.A.; Gopal, M.; Dharmaraj, S.; Chen, W.-H.; Kothari, R.; Manasa, I.; Park, J.H.; et al. Recent developments in biorefining of macroalgae metabolites and their industrial applications—A circular economy approach. Bioresour. Technol. 2022, 359, 127235.
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2021, 806, 151387.
- Zili, F.; Bouzidi, N.; Ammar, J.; Zakhama, W.; Ghoul, M.; Sayadi, S.; Ben Ouada, H. Mixotrophic cultivation promotes growth, lipid productivity, and P.U.F.A. production of a thermophilic Chlorophyta strain related to the genus Graesiella. J. Appl. Phycol. 2017, 29, 35–43.
- Jareonsin, S.; Pumas, C. Advantages of Heterotrophic microalgae as a host for phytochemicals production. Front. Bioeng. Biotechnol. 2021, 9, 628597.
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of polyunsaturated fatty acids and lipids from autotrophic, mixotrophic and heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791.
- Sim, S.J.; Joun, J.; Hong, M.E.; Patel, A.K. Split mixotrophy: A novel cultivation strategy to enhance the mixotrophic biomass and lipid yields of Chlorella protothecoides. Bioresour. Technol. 2019, 291, 121820.
- Varvoutis, G.; Lampropoulos, A.; Mandela, E.; Konsolakis, M.; Marnellos, G.E. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies 2022, 15, 4790.
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and Micro Algae in Pollution Control and Biofuel Production—A Review. ChemBioEng Rev. 2020, 7, 18–33.
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585.
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: The applications of high-rate algal ponds (H.R.A.P.s) and algal turf scrubber (A.T.S.). J. Environ. Manag. 2021, 296, 113193.
- da Rosa, M.D.H.; Alves, C.J.; dos Santos, F.N.; de Souza, A.O.; Zavareze, E.d.R.; Pinto, E.; Noseda, M.D.; Ramos, D.; de Pereira, C.M.P. Macroalgae and Microalgae Biomass as Feedstock for Products Applied to Bioenergy and Food Industry: A Brief Review. Energies 2023, 16, 1820.
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18.
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354.
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural features and rheological properties of a sulfated xylogalactan-rich fraction isolated from tunisian red seaweed. Jania adhaerens. Appl. Sci. 2020, 10, 1655.
- Aumeerun, S.; Soulange-Govinden, J.; Driver, M.F.; Rao, A.R.; Ravishankar, G.A.; Neetoo, H. Macroalgae and Microalgae. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; p. 207.
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600.
- Kraan, S. Algal Polysaccharides, Novel applications and outlook. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012; Chapter 22; pp. 489–524.
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152.
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R.
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117.
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67.
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631.
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426.
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458.
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.-L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an antiwrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224.
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8.
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The potential of sulfated polysaccharides isolated from the brown seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724.
- Xue, C.-H.; Fang, Y.; Lin, H.; Chen, L.; Li, Z.-J.; Deng, D.; Lu, C.-X. Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2001, 13, 67–70.
- Usov, A.I.; Zelinsky, N.D. Chemical Structures of Algal Polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 23–86. ISBN 978-0-85709-512-1.
- Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172.
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseca, T.N.; Ermakova, S.P.; Lee, Y.P. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134.
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in Zebrafish. Mar. Drugs 2020, 18, 316.
- Wang, L.; Oh, J.-Y.; Kim, Y.-S.; Lee, H.-G.; Lee, J.-S.; Jeon, Y.-J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427.
- Thomas, N.V.; Kim, S. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164.
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459.
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and M.A.P.K. signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759.
- Ali Karami, M.; Sharif Makhmalzadeh, B.; Pooranian, M.; Rezai, A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: An in vivo evaluation of skin protection against UVB. Pharm. Dev. Technol. 2021, 26, 209–219.
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Academic Press: London, UK, 2016; pp. 223–247.
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644.
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774.
- Hentati, F.; Ursu, A.V.; Pierre, G.; Delattre, C.; Bogdan, T.; Abdelkafi, S.; Gholamereza, D.; Tanase, D.; Michaud, P. Production, extraction and characterization of alginates from seaweeds. In Handbook of Algal Technologies and Phytochemicals; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press (Taylor & Francis Group, Royaume-Uni): Boca Raton, FL, USA, 2019; pp. 33–42.
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713.
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597.
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144.
- Valado, A.; Pereira, M.; Amaral, M.; Cotas, J.; Pereira, L. Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases. Nutraceuticals 2022, 2, 441–454.
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and its applications. In Polysaccharides; Springer: Cham, Switzerland, 2014; pp. 1–37.
- Knutsen, S.; Myslabodski, D.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169.
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528.
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076.
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydary, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.-H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Altern. Complement. Med. 2021, 17, 20190203.
- Miladi, R.; Manghisi, A.; Minicante, S.A.; Genovese, G.; Abdelkafi, S.; Morabito, M. A DNA barcoding survey of Ulva (Chlorophyta) in Tunisia and Italy reveals the presence of the overlooked alien U. ohnoi. Cryptogam. Algol. 2018, 39, 85–107.
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774.
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702.
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42.
- Briand, X.; Cluzet, S.; Dumas, B.; Esquerre-Tugaye, M.T.; Salamagne, S. Use of Ulvans as Activators of Plant Defence and Resistance Reactions against Biotic and Abiotic Stresses. U.S. Patent 0232494 A1, 13 October 2005.
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313318.
- Kidgell, J.T.; Carnachan, S.M.; Magnusson, M.; Lawton, R.J.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Glasson, C.R.K. Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohyd. Polym. 2021, 264, 118010.
- Fournière, M.; Bedoux, G.; Lebonvallet, N.; Lescchiera, R.; Goff-Pain, C.L.; Bourgougnon, N.; Latire, T. Poly-and oligosaccharide ulva sp. Fractions from enzyme-assisted extraction modulate the metabolism of extracellular matrix in human skin fibroblasts: Potential in antiaging dermo-cosmetic applications. Mar. Drugs 2021, 19, 156.
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry-An Update. Cosmetics 2017, 5, 2.
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757.
- Moser, G.A.O.; Barrera-Alba, J.J.; Ortega, M.J.; Alves-de-Souza, C.; Bartual, A. Comparative characterization of three Tetraselmis chui (Chlorophyta) strains as sources of nutraceuticals. J. Appl. Phycol. 2022, 34, 821–835.
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987.
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085.
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs 2020, 18, 142.
- Teixeira, T.R.; Santos, G.S.; Turatti, I.C.C.; Paziani, M.H.; von Zeska Kress, M.R.; Colepicolo, P.; Debonsi, H.M. Characterization of the lipid profile of Antarctic brown seaweeds and their endophytic fungi by gas chromatography–mass spectrometry (G.C.–M.S.). Polar Biol. 2019, 42, 1431–1444.
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39.
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-Obesity Effects of Macroalgae. Nutrients 2020, 12, 2378.
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96.
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144.
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52.
- Law, S.Q.; Mettu, S.; AshokKumar, M.; Scales, P.J.; Martin, G.J. Emulsifying properties of ruptured microalgae cells: Barriers to lipid extraction or promising biosurfactants? Colloids Surf. B Biointerfaces 2018, 170, 438–446.
- da Silva, M.E.T.; Leal, M.A.; de Oliveira Resende, M.; Martins, M.A.; dos Reis Coimbra, J.S. Scenedesmus Obliquus Protein Concentrate: A Sustainable Alternative Emulsifier for the Food Industry. Algal Res. 2021, 59, 102468.
- Hernandez, E.M. Pharmaceutical and Cosmetic Use of Lipids. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2020; pp. 1–28.
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552.
- Admassu, H.; Abdalbasit, M.; Gasmalla, A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16.
- Dini, I.; De Biasi, M.-G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483.
- Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788.
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Toy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140.
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical composition and potential practical application of 15 red algal species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489.
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Medic. 2014, 34, 796–803.
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (M.A.A.s): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63.
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586.
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.U.V. Photoprotectants from Algae—Synthesis and Bio-Functionalities. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38.
- Suh, S.S.; Oh, S.K.; Lee, S.G.; Kim, I.C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264.
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Antiinflammatory, and Antiaging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222.
- Gianeti, M.D.; Maia Campos, P.M.B.G. Efficacy evaluation of a multifunctional cosmetic formulation: The benefits of a combination of active antioxidant substances. Molecules 2014, 19, 18268–18282.
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17.
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326.
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384.
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777.
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 278–334.
- Dini, I.; Laneri, S. Spices, Condiments, extra virgin olive oil and aromas as not only flavorings, but precious allies for our wellbeing. Antioxidants 2021, 10, 868.
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2021, 42, 23–45.
- Shrestha, A.; Pradhan, R.; Ghotekar, S.; Dahikar, S.; Marasini, B.P. Phytochemical Analysis and Anti-Microbial Activity of Desmostachya Bipinnata: A review. J. Med. Chem. Sci. 2021, 4, 36–41.
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847.
- Charoensiddhi, S.; Franco, C.; Su, P.; Zhang, W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J. Appl. Phycol. 2015, 27, 2049–2058.
- Chang, M.Y.; Byon, S.H.; Shin, H.C.; Han, S.E.; Kim, J.Y.; Byun, J.Y.; Lee, J.D.; Park, M.K. Protective effects of the seaweed phlorotannin polyphenolic compound dieckol on gentamicin-induced damage in auditory hair cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 31–36.
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112.
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37.
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-Binding Inhibition of Tyrosinase by Ecklonia cava Phlorotannins. Mar. Drugs 2019, 17, 359.
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135.
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective Effect of Diphlorethohydroxycarmalol against Ultraviolet B Radiation-Induced D.N.A. Damage by Inducing the Nucleotide Excision Repair System in HaCaT Human Keratinocytes. Mar. Drugs 2015, 13, 5629–5641.
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Yang, H.M.; Kim, A.D.; Jeon, Y.J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316.
- Barbosa, M.; Lopes, G.; Andrade, P.-B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171.
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478.
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658.
- Kim, E.K.; Tang, Y.; Kim, Y.S.; Hwang, J.W.; Choi, E.J.; Lee, J.H.; Lee, S.H.; Jeon, Y.J.; Park, P.J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs 2015, 13, 1785–1797.
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268.
- Lee, Y.J.; Park, J.H.; Park, S.A.; Joo, N.R.; Lee, B.H.; Lee, K.B.; Oh, S.M. Dieckol or phlorofucofuroeckol extracted from Ecklonia cava suppresses lipopolysaccharide-mediated human breast cancer cell migration and invasion. J. Appl. Phycol. 2020, 32, 631–640.
- Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369.
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988.
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2007; pp. 255–261.
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87.
- Kumar, L.R.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185.
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709.
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129.
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170.
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130.
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of Antioxidant, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase of The Extract and Fraction From Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188.
- Bak, S.S.; Sung, Y.K.; Kim, S.K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 789–793.
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52.
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329.
- Ryu, B.; Ahn, B.-N.; Kang, K.-H.; Kim, Y.-S.; Li, Y.-X.; Kong, C.-S.; Kim, S.-K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B 2015, 153, 352–357.
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.H.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N.; Ngo, D.N. The suppressive activity of fucofuroeckol-a derived from brown algal Ecklonia stolonifera okamura on UVB-induced mast cell degranulation. Mar. Drugs 2018, 16, 1.
- Joe, M.; Kim, S.; Choi, H.; Shin, W.; Park, G.; Kang, D.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739.
- Le, Q.; Li, Y.; Qian, Z.; Kim, M.; Kim, S. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176.
- Sugiura, Y.; Kinoshita, Y.; Misumi, S.; Yamatani, H.; Katsuzaki, H.; Hayashi, Y.; Murase, N. Correlation between the seasonal variations in phlorotannin content and the antiallergic effects of the brown alga Ecklonia cava subsp. stolonifera. Algal Res. 2021, 58, 102398.
- Handajani, F.; Prabowo, S. Sargassum duplicatum extract reduced artritis severity score and periarticular tissue matrix metalloproteinase-1 (MMP-1) expression in ajuvan artritis exposed to cold stressor. Syst. Rev. Pharm. 2020, 11, 302–307.
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292.
- Dong, H.; Wang, L.; Guo, M.; Stagos, D.; Giakountis, A.; Trachana, V.; Lin, X.; Liu, Y.; Liu, M. Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants 2022, 11, 786.
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106.
- Ryu, Y.S.; Fernando, P.D.S.M.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine compound 3-bromo-4,5-dihydroxybenzaldehyde protects skin cells against oxidative damage via the Nrf2/HO-1 pathway. Mar. Drugs 2019, 17, 234.
- Cho, S.H.; Heo, S.J.; Yang, H.W.; Ko, E.Y.; Jung, M.S.; Cha, S.H.; Ahn, G.; Jeon, Y.J.; Kim, K.N. Protective effect of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii harvey against hydrogen peroxide-induced oxidative stress in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203.
- Hyun, Y.J.; Piao, M.J.; Zhang, R.; Choi, Y.H.; Chae, S.; Hyun, J.W. Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol. Environ. Saf. 2012, 83, 71–78.
- Kim, K.; Hyun, Y.; Hewage, S.R.; Piao, M.; Kang, K.; Kang, H.; Koh, Y.; Ahn, M.; Hyun, J. 3-bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15, 291.
- Xu, X.L.; Yin, L.Y.; Gao, J.H.; Gao, L.J.; Song, F.H. Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem. Biodivers. 2015, 11, 807–811.
- Xu, X.L.; Yin, L.Y.; Wang, Y.H.; Wang, S.Y.; Song, F.H. A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat. Prod. Res. 2012, 27, 723–726.
- Rajasulochana, P.; Krishnamoorthy, P.; Dhamotharan, R. Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycus sp. Int. J. Pharm. Biol. Sci. 2012, 3, 173–186.
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoo, E.S. Antiinflammatory effect of 3-bromo-4,5-dihydroxybenzaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332.
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme. Inhib. Med. Chem. 2017, 32, 403–425.
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706.
- Mikami, D.; Kurihara, H.; Kim, S.M.; Takahashi, K. Red algal bromophenols as glucose 6-phosphate dehydrogenase inhibitors. Mar. Drugs 2013, 11, 4050–4057.
- Mikami, D.; Kurihara, H.; Ono, M.; Kim, S.M.; Takahashi, K. Inhibition of algal bromophenols and their related phenols against glucose 6-phosphate dehydrogenase. Fitoterapia 2016, 108, 20–25.
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844.
- Sava, C.; Sibru, R. Analytical study of the determination of flavonoids in Black Sea algae. Ovidius Univ. Ann. Chem. 2010, 21, 29–34.
- Xiao, X.; Li, C.; Huang, H.; Lee, Y.P. Inhibition effect of natural flavonoids on red tide alga Phaeocystis globosa and its quantitative structure-activity relationship. Environ. Sci. Pollut. Res. 2019, 26, 23763–23776.
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203.
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štĕrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965.
- Catarino, M.D.; Silva-Reis, R.; Chouh, A.; Silva, S.; Braga, S.S.; Silva, A.M.S.; Cardoso, S.M. Applications of Antioxidant Secondary Metabolites of Sargassum spp. Mar. Drugs 2023, 21, 172.
More
