The antibacterial hydrogel coating interacts with organic and inorganic components as a biocompatible surface modifier, and the coating acts as a buffer between biomaterials and human tissues, making the biphasic interface of the material more stable and flexible and meeting the various needs of human tissue repair. The two key advantages of hydrogel coatings are as follows: Firstly, the coating can be firmly attached to the surface through chemical crosslinking and various anchoring reactions. Secondly, the coatings can attach to almost all kinds of materials, such as precious metals, oxides, polymers, and ceramics. Hydrogel coatings have excellent prospects for application, simple processing, stable performance, and wide application. The current research difficulties include the following: Firstly, the preparation method of antibacterial hydrogel coatings needs to be improved. Although the graft density of surface-initiated graft crosslinking polymerization is high, the initiator needs to be grafted to the surface, and the preparation process is relatively complex. The method of fixing the hydrogel coating to the substrate surface may result in uneven coverage of the hydrogel coating to the substrate surface due to the steric hinderance of the graft chain. Secondly, greater attention should be given to the study of the chemical stability of hydrogel coatings, including swelling, durability, degradability, mechanical properties, etc., which are important for the long-term effect of antibacterial hydrogel coatings on the human body. For example, swelling could be a problem for the coating of tubular medical devices, as the large swelling degree of hydrophilic hydrogels might block the tube. Finally, sterilization has been reported as an issue for most hydrogel coatings.
- hydrogel coatings
- antibacterial property
- biomaterials
1. Introduction
In the current biomedical field, medical devices, such as catheters, hernia nets, implants, and wound dressings, are often adhered by bacteria, leading to varying degrees of infection and posing a threat to the health of patients [1]. Once bacteria adhere to the surface of the substrate, they will rapidly form a biofilm, which attracts more bacteria, affecting the antibacterial effect of the immune system [2]. Therefore, the prevention of bacterial infection in the process of biomaterial implantation has become the focus of researchers. Surface coating or modification, which preserves the original properties of the material and changes only the surface properties, has been recognized as a promising strategy for introducing antibacterial efficacy into biomaterials. Some bionic surface morphologies with high aspect ratios are effective against colonization by bacteria, although the mechanism of antibacterial activity is not clear [3]. For methods of tailoring surface chemistry, substrates can be chemically modified or physically coated with a variety of antibacterial substances, including polymers, functional groups, inorganic nanoparticles, hydrogels, and antibiotics [4]. Among these bactericidal materials, hydrogel coating has many advantages and has been widely studied.
2. Preparation Methods of Hydrogel Coatings
Hydrogels are prepared by chemical and physical crosslinking, but it is challenging to fix physically crosslinked hydrogels on the surface of materials due to the lack of binding sites for binding into the three-dimensional network [5]. In addition, the poor mechanical properties of physically crosslinked hydrogels compared to chemically crosslinked hydrogels limit the application of physically crosslinked hydrogels as durable coating materials [6]. The chemically crosslinked hydrogels can be gelatinized by monomer polymerization or conjugation reactions between polymer chains, whereby we can attach these hydrogels to the material surface in various ways to form stable hydrogel coatings [7]. The general strategies can be divided into three types: The first is surface-initiated graft crosslinking polymerization. The second is anchoring the hydrogel coating to the substrate surface. Third is the LbL self-assembly technique to coat crosslinked hydrogels.
2.1. Surface-Initiated Graft Crosslinking Polymerization
Free radical polymerization is a crucial way to prepare polymeric materials. The polymerization reaction is initiated by active radicals located on the surface of the substrate, so the adhesion of hydrogels can be promoted by generating active radicals on the substrate surface and introducing grafting sites on the surface [8].
2.2. Anchoring the Hydrogel Coating to the Substrate Surface
The preparation of stable polymer brushes on the material surface is possible by initiating graft-crosslinking polymerization on the substrate surface, which introduces a high density of reaction sites. However, the reaction efficiency is low, and the preparation of hydrogel coatings on the material surface does not require too many reaction sites. In general terms, the hydrogel can be thought of as a large molecule; immobilization on the material surface improves the coating binding stability and reaction efficiency.
2.3. LbL Self-Assembly Technique to Coat Crosslinked Hydrogels
The layer-by-layer self-assembly technique may prepare coatings on substrate surfaces through alternating deposition methods [9], and the coatings can be attached mainly by hydrogen bonding, electrostatic interactions, and charge transfer interactions. Because of the low stability of such noncovalent bonding forces, each layer can be deposited conjugately by covalent bonding in the preparation of hydrogel coatings. The LbL self-assembly technique is more suitable for preparing ultrathin hydrogel coatings. The substrate surface needs pretreatment, and the covalent attachment of the layer to the substrate surface may be realized by grafting functional groups [10]. For example, the surface of the silicon wafer undergoes functionalization through reactants containing dopamine groups; an ultrathin hydrogel coating can be prepared by depositing alternating polyacrylic acid (PAA) and chitosan quaternary ammonium salts using the LbL self-assembly technique. The coating surface is positively charged and effectively inhibits bacterial adhesion.
3. Hydrogel Coatings in Biomedical Antibacterial Applications
The following three antibacterial methods are the main methods for material surface modification by hydrogel coatings: The first is bacterial repellence and inhibition. The second is the contact surface killing of bacteria. The third is the release of antibacterial agents (Figure 1).
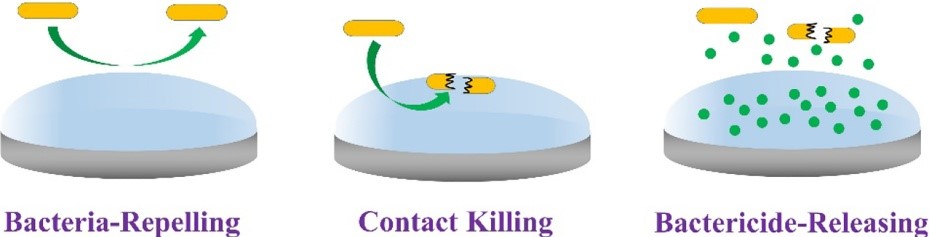
Figure 1. Strategies for antibacterial hydrogel coatings. Copyright 2020, Elsevier.
3.1. Bacterial Repellence and Inhibition
In the earliest stage of bacterial biofilm formation, bacterial adhesion on the surface is reversible, so the introduction of hydrogel coatings that repell bacterial adhesion is the most direct antibacterial method. The hydrogel coating prepared by this method has better biocompatibility [11]. When biomaterials are implanted into the body, proteins tend to absorb nonspecifically on the surface, which promotes bacterial adhesion; thus, the repellent hydrogel coating should effectively inhibit the nonspecific absorption of proteins. Substances with hydrogen bonding acceptors and hydrophilic polar functional groups may inhibit the nonspecific absorption of proteins; they may form hydrogen bonds with water molecules in aqueous media and form a highly hydrated layer on the polymer surface to effectively achieve antibacterial properties. Polyethylene glycols (PEG) [12], polyvinyl alcohols (PVA) [13], polyacrylates [14], amphiphilic polymers [15], polysaccharides, and other hydrophilic substances are widely used as raw materials in the field of antibacterial hydrogels. Next, the applications of hydrogel coatings containing the above substances in biomaterials are introduced [16].
3.2. Contact Surface Killing of Bacteria
Researches show that the combination of bactericidal compounds and hydrogel coatings can effectively kill bacteria. Different from the passive antibacterial mechanism of bacterial-repelling hydrogel coatings, the bactericidal hydrogel coating can actively kill bacteria by destroying the cell membrane of bacteria, thus preventing the propagation of bacteria and achieving effective antibacterial activity. The bactericidal compounds commonly used generally contain cations and hydrophobic groups, and since bacteria have negative charges, they can be adsorbed by the cations of the bactericidal compounds. The hydrophobic groups of bactericides may also damage the lipid composition of the bacterial membranes. Some widely used bactericides are antimicrobial peptides (AMPs) and quaternary ammonium compounds (QACs) [17].
3.3. Release of Antibacterial Agents
The three-dimensional network structure of hydrogels can be loaded with antibiotics, AMPs, cationic polymers, silver ions, copper ions, antibacterial drugs, and other bactericidal compounds to enhance the antibacterial properties of the biomaterials. Compared with the above two methods, this method is more flexible and controllable, repelling bacteria and killing bacteria via released antibacterial agents. However, the preparation process is relatively complex and requires consideration of the release rate and concentration of the antimicrobial agents. For example, Hoque et al. prepared a biocompatible hydrogel using dextran methacrylate (Dex-MA) as a monomer and encapsulated it with a small molecular cationic biocide by in situ loading during photopolymerization. The hydrogels showed a sustained release of biocide and displayed 100% activity against methicillin-resistant Staphylococcus aureus (MRSA) for an extended period of time (until day 5) [18].
Due to the limitation of the surface hydrogel coating function, the coated antibacterial substance will become depleted gradually and cannot maintain an excellent antibacterial effect for a long period. Therefore, the release rate of antibacterial substances should be reasonably controlled. Thus, an intelligently controlled release coating structure can be prepared to release antibacterial substances under specific conditions (pH, light reaction, temperature, and REDOX reaction) to kill bacteria and free bacteria attached to the material surface.
4. Conclusions
This paperntry provides a detailed review of the application of hydrogel coatings in biomedical antibacterial applications and introduces the principles of adhesion on the surface of materials and antibacterial strategies. Hydrogels can be attached to the surface of biomaterials in three ways: The first is surface-initiated graft crosslinking polymerization. The second is anchoring the hydrogel coating to the substrate surface. The third is the LbL self-assembly technique to coat crosslinked hydrogels. Hydrogel coatings’ antibacterial strategies are divided into three types: The first type is bacteria repellence and inhibition. The second type is the contact surface killing of bacteria. The third type is the release of antibacterial agents.
References
- Bao, Y.; Li, Z.; Li, Y.; Chen, T.; Cheng, Y.; Xu, M. Recent Advances of Biomedical Materials for Prevention of Post-ESD Esophageal Stricture. Front. Bioeng. Biotechnol. 2021, 9, 792929, 10.3389/fbioe.2021.792929.
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280, 10.1016/j.cis.2020.102280.
- Zhang, F.; Cheng, Z.; Ding, C.; Li, J. Functional biomedical materials derived from proteins in the acquired salivary pellicle. J. Mater. Chem. B 2021, 9, 6507-6520, 10.1039/D1TB01121A.
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295-304, 10.1016/j.tibtech.2013.01.017.
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014, 10.1126/science.1069210.
- Beaman, H.T.; Howes, B.; Ganesh, P.; Monroe, M.B.B. Shape memory polymer hydrogels with cell-responsive degradation mechanisms for Crohn’s fistula closure. J. Biomed. Mater. Res. A 2022, 110, 1329-1340, 10.1002/jbm.a.37376.
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6-22, 10.1016/j.actbio.2017.11.003.
- Zhang, Z.; Zhang, Z.; Hong, Z. Unscented Kalman Filter-Based Robust State and Parameter Estimation for Free Radical Polymerization of Styrene with Variable Parameters. Polymers 2022, 14, 973, 10.3390/polym14050973.
- Liu, Q.; Gao, S.; Zhao, Y.; Tao, W.; Yu, X.; Zhi, M. Review of layer-by-layer self-assembly technology for fire protection of flexible polyurethane foam. J. Mater. Sci. 2021, 56, 9605–9643, 10.1007/s10853-021-05904-3.
- Zhu, D.; Guo, D.; Zhang, L.; Tan, L.; Pang, H.; Ma, H.; Zhai, M. Non-enzymatic xanthine sensor of heteropolyacids doped ferrocene and reduced graphene oxide via one-step electrodepo-sition combined with layer-by-layer self-assembly technology. Sens. Actuators B Chem. 2019, 281, 893–904, 10.1016/j.snb.2018.10.151.
- Hu, Q.; Liu, Y.; Pan, Y.; Wang, Y.; Jiang, L.; Lin, H.; Cheng, Y.; Xu, C.; Lin, D.; Cheng, H.; et al. Assessments of ionic release and biocompatibility of Co-Cr and CP-Ti produced by three different manufacturing techniques. Mater. Today Commun 2022, 30, 103100, 10.1016/j.mtcomm.2021.103100.
- Hu, F.; Lu, H.; Xu, G.; Lv, L.; Chen, L.; Shao, Z. Carbon quantum dots improve the mechanical behavior of polyvinyl alcohol/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 2022, 139, e52805, 10.1002/app.52805.
- Zhang, M.-K.; Ling, X.-H.; Zhang, X.-H.; Han, G.-Z. A novel alginate/PVA hydrogel -supported Fe3O4 particles for efficient heterogeneous Fenton degradation of organic dyes. Colloids Surf. Physicochem. Eng. Asp. 2022, 652, 129830, 10.1016/j.colsurfa.2022.129830.
- Patrick, D.K.; Karasawa, A.; Sonoyama, N. Sodium Polyacrylate Hydrogel Electrolyte Hybridized with Layered Double Hydroxide for Solid-State NiCo/Zinc Battery. J. Electrochem. Soc. 2022, 169, 040559, 10.1149/1945-7111/ac65b4.
- Nakano, H.; Kakinoki, S.; Iwasaki, Y. Long-lasting hydrophilic surface generated on poly(dimethyl siloxane) with photoreactive zwitterionic polymers. Colloids Surf. B Biointerfaces 2021, 205, 111900, 10.1016/j.colsurfb.2021.111900.
- Mondal, P.; Chatterjee, K. Injectable and self-healing double network polysaccharide hydrogel as a minimally-invasive delivery platform. Carbohydr. Polym. 2022, 291, 119585, 10.1016/j.carbpol.2022.119585.
- Li, R.; He, S.; Yin, K.; Zhang, B.; Yi, Y.; Zhang, M.; Pei, N.; Huang, L. Effects of N-terminal modifications on the stability of antimicrobial peptide SAMP-A4 analogues against protease degradation. J. Pept. Sci. 2021, 27, e3352, 10.1002/psc.3352.
- Hoque, J.; Haldar, J. Direct Synthesis of Dextran-based Antibacterial Hydrogels for Extended Release of Biocides and Eradication of Topical Bio-films. ACS Appl. Mater. Interfaces 2021, 9, 15975–15985.
