The phytochrome (phy) system of plants with the main phyA and phyB controls their development beginning from seed germination to fruiting and senescence. The regulation reactions are categorized into three modes—the very low and low fluence responses (VLFR and LFR) and the high irradiance responses (HIR). The phyA is unique among the other phytochromes; it is major in etiolated seedlings and light-labile, and mediates all the three photoresponse modes. The phyB is light-stable, dominates in deetiolated plants, and performs the LFR. The phyA is itself heterogeneous which may explain its functional complexity. It comprises two native types, phyA′ and phyA″, the products of post-translational modification of the molecule at the N-terminus, possibly, via serine phosphorylation. This alters chromophore-apoprotein interactions resulting in the different photochemical, phenomenological, and functional properties of the two phyA pools. The phyA′ is major, water-soluble, and light-labile; the phyA″ is minor, amphiphilic, and relatively light-stable. The phyA′ mediates the VLFR whereas the water-soluble fraction of phyA″ is responsible for the HIR and LFR, the processes taking place in the nucleus. The membrane- (protein-) associated fraction of phyA” is likely to participate in the cytoplasmic photoregulation processes. The phyA pools' functions—their mode, intensity and sign—depend on plant's species, genotype and organ/tissue. The evidence of the existence of the two distinct phyA types in a plant, and their physicochemical properties and their role in the phyA functioning are discussed.
- plants
- phytochrome A
- polymophism
- fluorescence
- photoreaction
- photoresponse
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98]
1. Introduction
In photobiology, investigations of the phytochrome system of plants are one of the most interesting and highly important problems both in fundamental and practical terms. The key role of the photoreceptor phytochrome is observed both in the individual development of the plant [1] and in the evolution of higher plants in general [2]. Investigations of phytochrome are obviously important for artificial light culture [3] and for nanotechnological and optogenetic purposes. It can be used as an effective light-driven molecular trigger, drug delivery carrier, and fluorescent reporter [4].
The discovery of the photoreceptor is associated with the detection of red (R)-induced/far-red (FR)-reversible physiological effects in plants (such as induction of germination, flowering, and fruiting) and R/FR reversible changes in the absorption spectra of plant tissues [5]. Research in these areas and by means of molecular biology and genetics led to the characterization of the photoreceptor as a molecule, to the phenomenological description of particular photoregulation plant reactions belonging to distinct photoresponse modes, and to the understanding of the mechanism of the light signal transduction from phytochrome. Very schematically, the chain of the transduction events can be described as follows. Phytochrome, a dimeric protein with a linear chromophore, bilin, upon absorption of light quanta undergoes photoisomerization reaction converting the initial red-light absorbing form Pr into the first stable at low temperatures (T) photorpoduct lumi-R, and following its dark transformations into the far-red absorbing physiologically active form Pfr. Pfr can return into Pr via a photochemical branch or thermally, thus completing the Pr↔Pfr photocycle [6][7][6,7]. This activation of phytochrome with the appearance of Pfr takes place in the cytoplasm, where the photoreceptor is synthesized in the Pr form in the dark, and leads to its transfer into the nucleus [8][9][8,9]. In the nucleus, Pfr deactivates phytochrome interacting factors (PIFs) blocking photomorphogenesis and activating factors (HY5), initiating it. Activation of photoresponsive genes leads to numerous phenomenological growth and development reactions [10][11][10,11]. Phytochrome is likely to be also engaged in several biophysical and biochemical events in the cytoplasm [12][13][14][15][16][12-16]. Resulting photophsyiological reactions are divided into (1) the very low fluence responses, VLFR, (2) the low fluence responses, LFR, and (3) the high irradiance responses, HIR [17][18][17,18].
It was widely accepted that plant’s responses were mediated by one molecular phytochrome species. Subsequently, however, compelling evidence for the existence of distinct phytochromes was obtained—the detection of (a) light-labile type 1 and light-stable type 2 phytochromes [19], (b) immunochemically distinct pools [20], (c) species differing by fluorescent and photochemical parameters [21][22][21,22], and, most importantly, (d) a divergent family of phytochrome genes with the two major gene products—phyA and phyB [1][23][24][1,23,24]. This implied that different phytochrome types might have unique photoregulatory roles—the VLFR and HIR were attributed to phyA, while the LFR, was to phyB. The phyA may also mediate the LFR. The light signal transduction from phyA and phyB were shown to proceeds, with different dynamics of the process, via separate chains and interacting partners [17][25][26][27][28][29][17,25–29]. The data are being accumulated, however, that phyA is itself structurally heterogeneous and can perform distinct photophysiological functions.
2. Phytochrome A Heterogeneity in the Cell: Chemically Distinct Species and Conformers
The means of phytochrome investigation in situ is limited because of very low concentration in plant tissues (<10−7 mol/L) and the presence in them of photosynthetic pigments interfering with measurements. In fact, there was only one spectroscopic method—difference absorption spectroscopy [30]. In [21][22][21,22], the fluorescence of phytochrome in-planta was detected and a sensitive and informative method of phytochrome investigation in its natural state in plant cells was developed . With its use phytochrome (the Pr form and the initial photorpoduct lumi-R) in the in etiolated plant tissues of monocots and dicots was for the first time characterized by fluorescence and photochemical parameters in the temperature (T) interval from 77-273 K (Figure 1). These are position of the fluorescence emission and excitation (absorption) maxima, activation and kinetic parameters of the Pr photoreaction and fluorescence quenching, relative phytochrome content in the sample, proportional to the Pr fluorescence intensity, and the extent of the Pr conversion into lumi-R at cryogenic T (Tc) and into Pfr at ambient T (Ta). All these parameters were shown to vary depending on plant species and genotype, its developmental state, organ/tissue used, growth conditions, and environmental factors, light pretreatment, in particular. This phenomenon was interpreted as a manifestation of the existence of at least two distinct phytochrome species, Pr′ and Pr″, characterized by different spectroscopic and photochemical parameters (Table 1). Most profound changes were observed in the ability of Pr to undergo photoconversion into lumi-R, measured as a relative Pr fluorescence decay after saturating red (R) preillumination at Tc (parameter γ1 that varied from approx. 0 to 0.5). This allowed for the determination of the Pr′/Pr″ proportion in the sample from the experimental γ1 value as described in [31]. The Pr′ can be characterized as a major and light-labile species, whereas Pr″ as a minor, saturable, and relatively light-stable (Table 1).
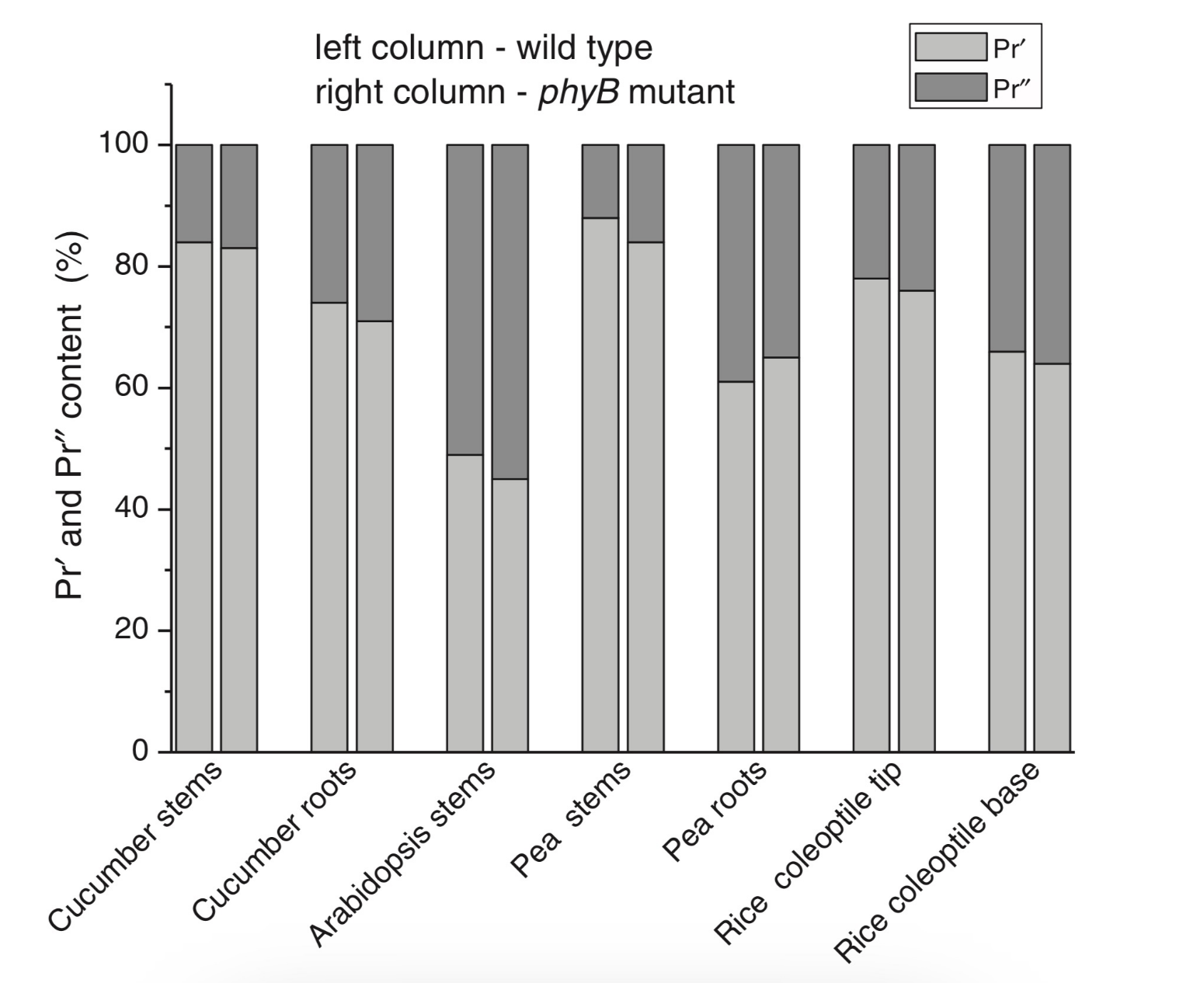
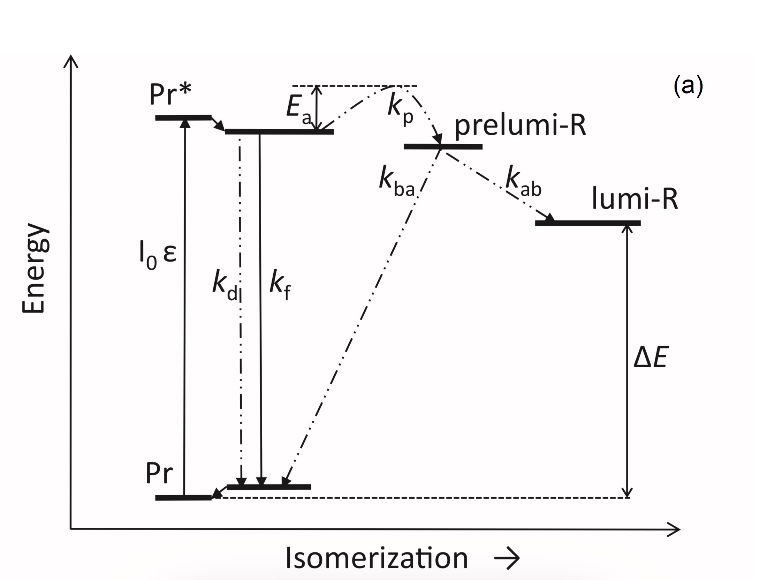
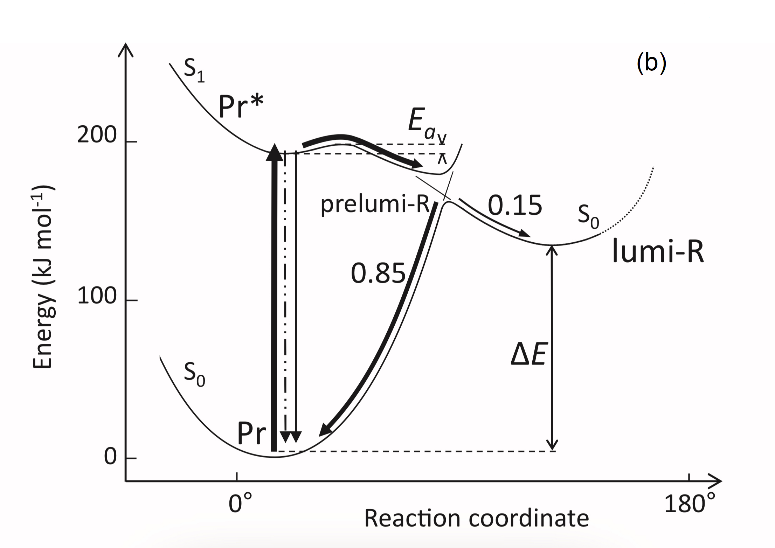
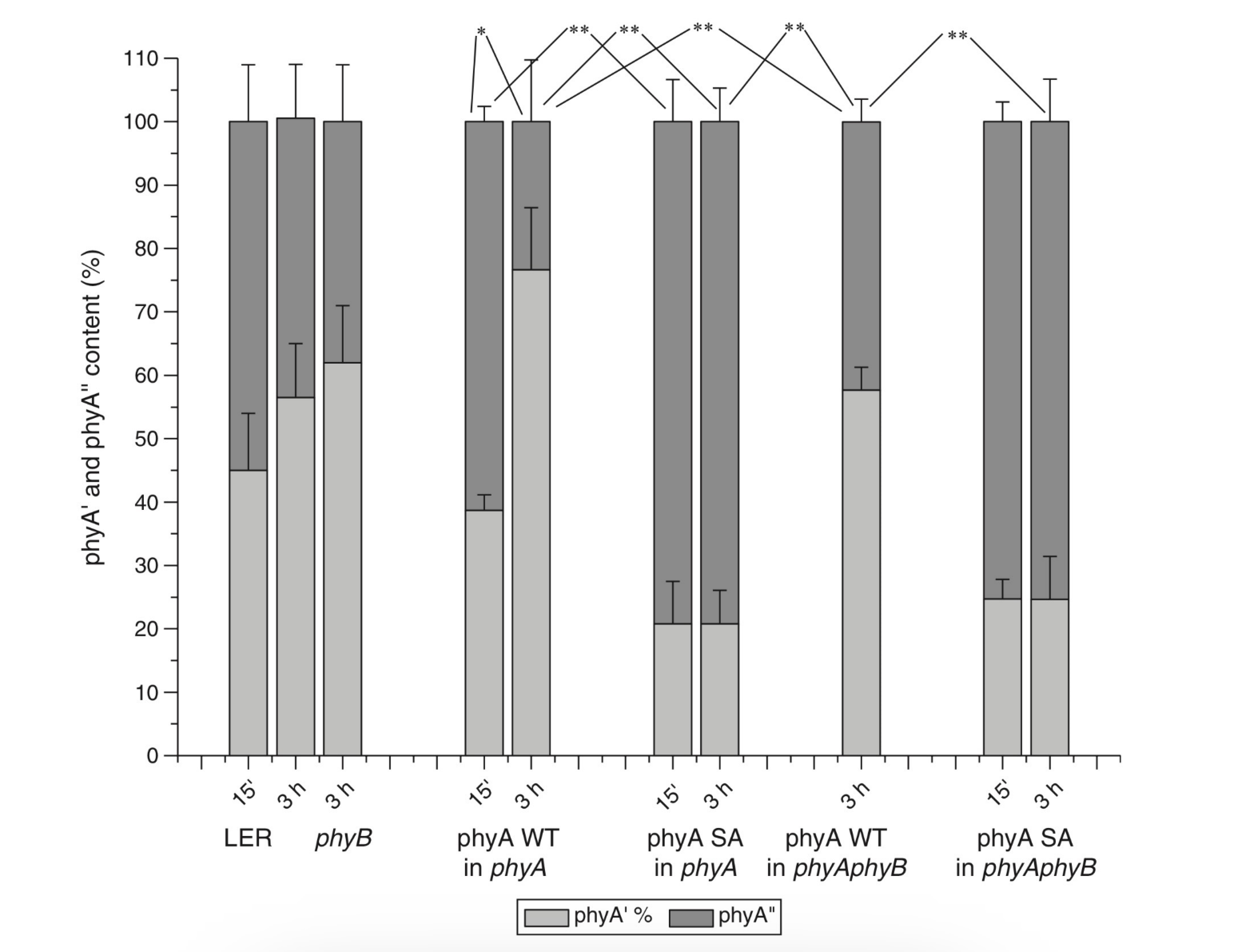
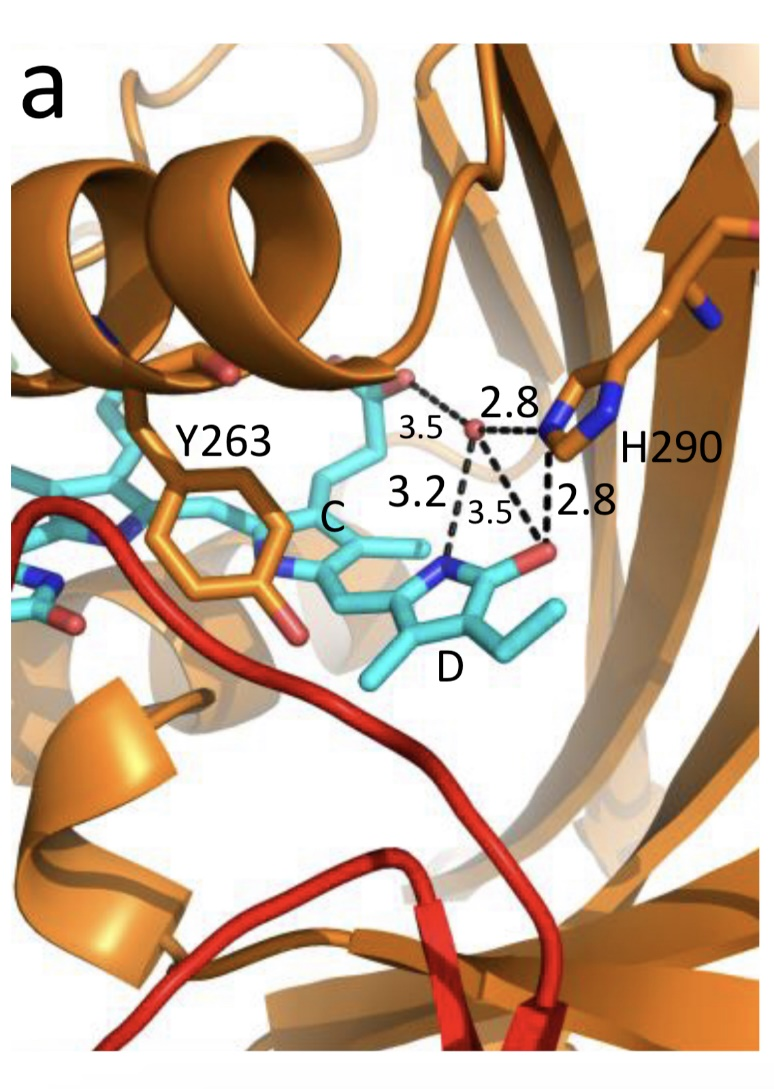
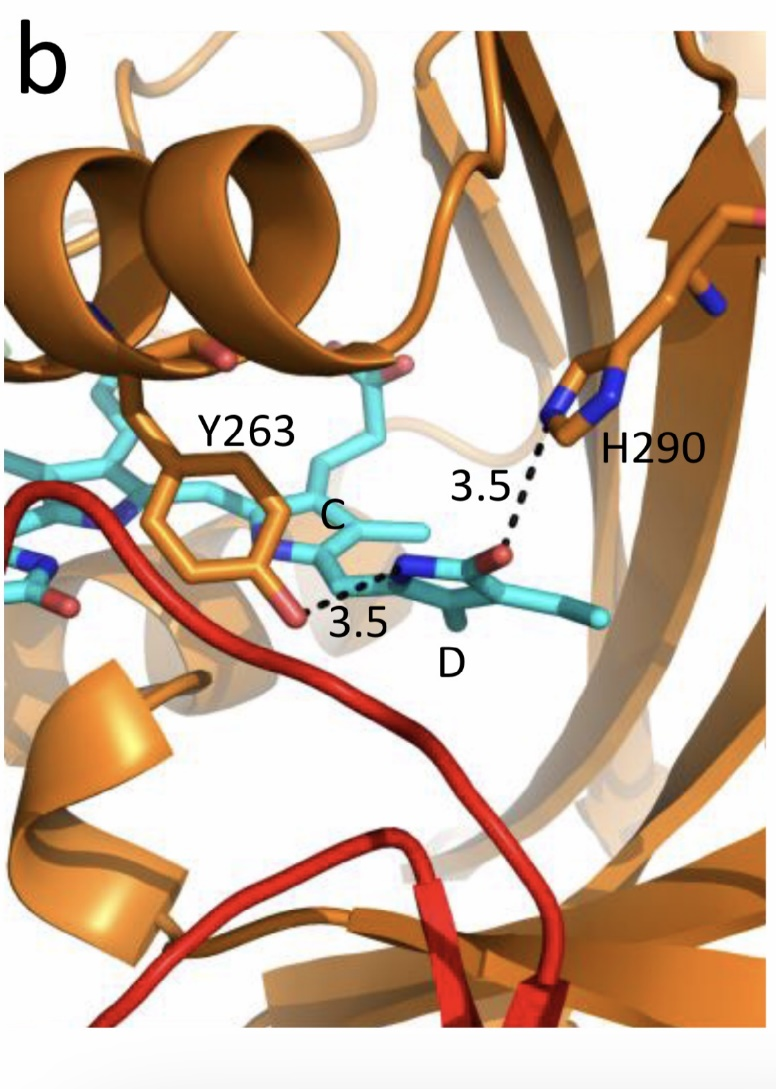
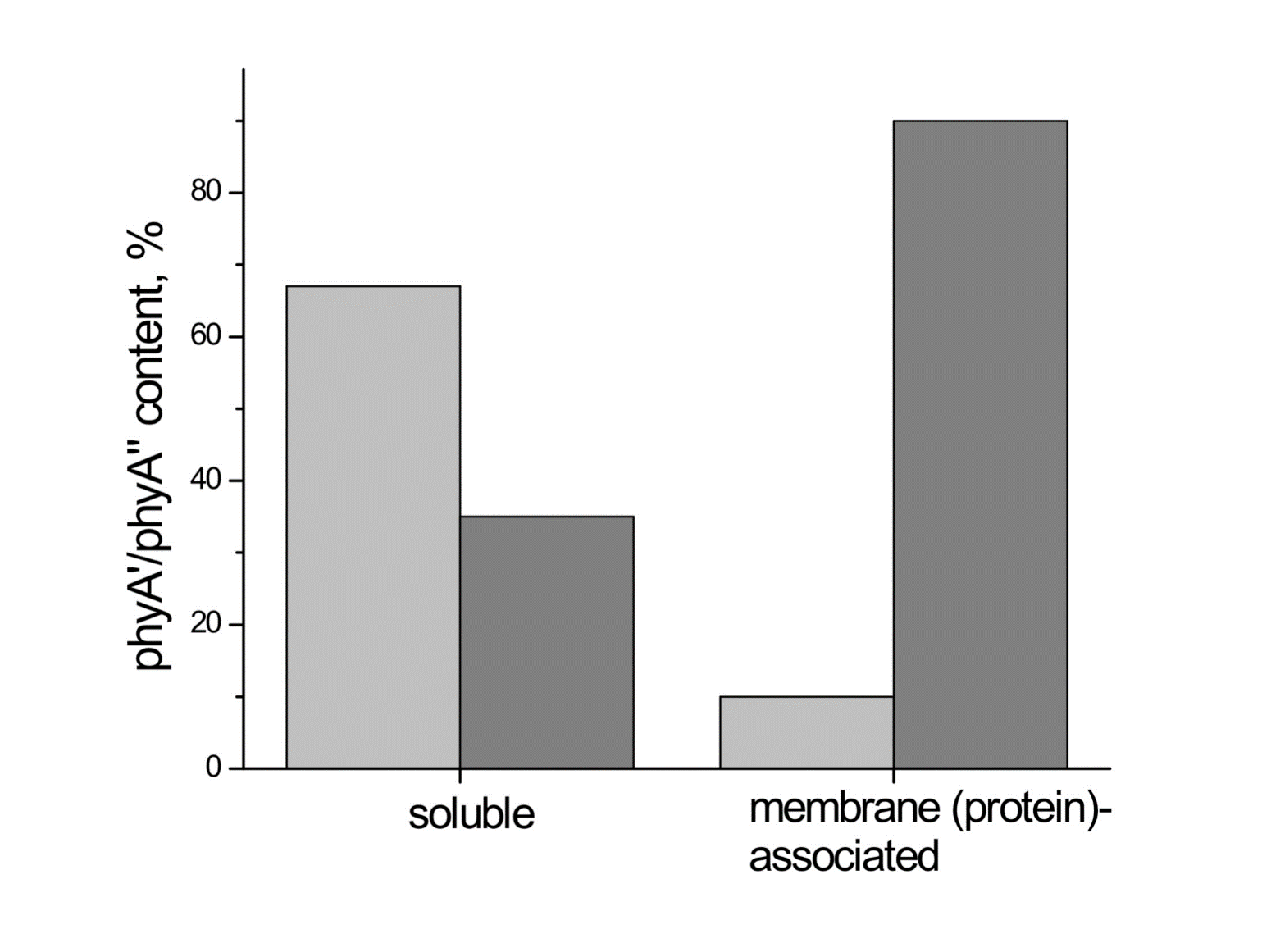
Figure 1. The low-temperature fluorescence method for phytochrome in-situ assay. To characterize the pigment, three fluorescence spectra of phytochrome were measured at 85 K and excitation wavelength at 633 nm: (1) in etiolated tissues (of wheat) when all phytochrome is in its Pr form; (2) in the same sample after saturating red illumination at 85 K partially converting Pr into lumi-R, the first stable photoproduct lumi-R; and (3) in the same sample after thawing at 273 K, saturating red illumination converting Pr into Pfr and freezing again at 85 K. Four major parameters were obtained from these spectra: position of the emission spectrum (λmax); total phytochrome content proportional to the fluorescence intensity ([Ptot]≈F0); extent of the Pr lumi-R photoconversion, equal to the relative fluorescence decline (γ1 = ∆F1/F0) and characterizing the initial photoreaction; and the extent of the Pr Pfr photoconversion (γ2 = ∆F2/F0), characterizing the whole phytochrome cycle. From [31][32][31,32]. Table 1. Two phenomenological phytochrome A types in mono- and dicotyledonous plants—Pr′ (phyA′) and Pr″ (phyA″). Parameter Phytochrome Type Pr′ (phyA′) Pr″ (phyA″) Position of emission/absorption maxima, λmax, nm Longer wavelength (685/672) Shorter wavelength (680/667) Half-band width, nm 22–24 30 Extent of Pr→lumi-R conversion at 85 K, γ1 0.49 ± 0.03 ≤0.05 Activation barrier in excited state, Ea, kJ/mol ≤1 ≥10 Extent of Pr→Pfr conversion at 273 K, γ2 0.80–0.85 0.75 Light lability Light labile Relatively light stable Hydrophilicity/Hydrophobicity Water–soluble Ambiquitous—soluble and membrane-(protein–) associated Content in etiolated tissues Major, variable Minor, saturated, conserved With the discovery of the two major phytochromes A and B—the major and light-labile phyA (type 1) and minor and light-stable phyB (type 2)—, it was tempting to assign the Pr′ and Pr″ species [33] to phyA and phyB, respectively. The picture proved, however, to be more complex than that. It was found that mutants lacking phyB—monocots (rice) and dicots (cucumber, Arabidopsis, pea)—contained practically the same amounts of Pr′ and Pr″ as the respective wild types suggesting that both Pr species belong to phyA differing presumably by post-translation modification (designated as phyA′ and phyA″) (Figure 2). Later on it was shown that phyB reveals properties close to those of phyA″ and belongs to the same Pr″ type [34]. The full-length Arabidopsis phyA expressed in P. pastoris and E. coli [35] (assembled in vivo with phycocyanobilin (PCB) or phytochromobilin (PΦB)) is similar to or identical with the phyA″ in plants. This demonstrates that the low-abundance–fraction plant phyA (phyA″) comes from the same gene as the major (phyA′) fraction. The heterogeneity was also observed in the case of phytochrome of lower plants (Adiantum phy1) [36]. To make the picture even more complex, it was found that phyA′ (photochemically active at Tc) is itself heterogeneous comprising distinct conformers [37]. Thus, it was concluded that the system of phytochromes in plants possesses three levels of complexity—(1) different gene products, (2) post-translationally modified products of one and the same gene, and (3) conformers within a distinct phytochrome species [7]. Figure 2. Relative content of the two phytochrome types, Pr′ and Pr″, differing by spectroscopic, photochemical and phenomenological properties (see Table 1), in etiolated dicotyledonous and monocotyledonous plants and their phyB-deficient mutants. Close similarity in the content of Pr′ and Pr″ between the wild type plants and the mutants strongly suggest that there exist two phyA species, phyA′ and phyA″, with the properties of Pr′ and Pr″ respectively (modified from [38][39][40][41][38–41]). The notion of the heterogeneity of phyA is supported by the investigations of the cyanobacterial phytochrome Cph1 which is considered a suitable model of plant phytochromes. Tthe fluorescence and photochemical characteristics of Cph1 were shown to be similar to those of the Pr′′ type (that is of phyA′′ and phyB). Two species with distinct fluorescence and photochemical characteristics and Ea but with relatively equal yields of the photoconversion at ambient T were also detected in the case of cyanobacterial Cph1 which are considered to be distinct conformers of the pigment [42]. Time-resolved and structural investigations of Cph1 in recent years have confirmed the notion of the heterogeneity of the ground state Pr population as a source of the complex kinetic and energetic processes in the phytochrome molecule . Thus, this phenomenon is not likely to be a unique feature of phyA (see discussion in [43]). The fluorescent and photochemical diversity of the phytochrome species can be interpreted in the framework of the scheme of the initial photoprocesses in photoisimerizing pigments (Figure 3) [33][42][33,42] (for detailed discussion, see reviews [7][43][7,43]). According to it, the photoisomerization, a single rotation around C15═C16 double bond in the excited Pr* state, has two major features which allow for the interpretation of the two phenomenological facts—first, the ability and inability of the two phyA species, respectively, to undergo the photochemical Pr→lumi-R conversion at Tc, and second, their almost identical quantum yields and extent of the Pr→Pfr phototransformation at Ta. These are, respectively, the existence of the activation barrier Ea in the excited Pr state (Pr*) for the photoreaction (it varies from hundreds J/mol for the Pr′ type and from several to tens kJ/mol for the Pr″ type [37][42][37,42]) the orthogonal prelumi-R “hot” ground state (real or virtual), where the branching of the photochemical routes takes place—direct productive prelumi-R→lumi-R or reverse unproductive prelumi-R→Pr. In this branching, the reverse process dominates thus lowering the yield of the Pr→Pfr photoconversion which is evaluated to be around 0.13–0.15 [6][7][42][6,7,42]. Figure 3. Energy level schemes of the photoreaction of the initial red-absorbing form (Pr) (Pr* is the excited state of Pr) into the first photoproduct (lumi-R) stable at low temperatures via a short-lived unstable orthogonal intermediate (prelumi-R) suggested for plant phytochrome (a) and for the cyanobacterial phytochrome Cph1 (b). At 85 K and saturating red illumination (λa = 632.8), there is a photoequilibrium between Pr and lumi-R determined by the rates of the forward (Pr→lumi-R) and reversed (lumi-R→Pfr) photoreactions. The activation barrier in the excited state, Ea for Pr and Ea′ for lumi-R, determines the photochemical properties of Pr and lumi-R and the extent of the Pr photoconversion to reach a photoequilibrium with lumi-R at low T (γ1 varies for different Pr species from 0 to 0.5). At ambient temperatures, this barrier is easily overcome and the extent of the Pr→Pfr photoconversion γ2 remains relatively constant, 0.75–0.85. (From [7][33][7,33]). (b) Hypothetical potential energy curves and quantum yields of the Pr photoreaction in Cph1 based on the scheme in (a). From [42]. The energy barrier Ea and the pattern of the branching of the conversion pathways at the prelumi-R point need to be interpreted in terms of the phytochrome molecular structure and chromophore-apoprotein interactions. Comparative analysis of the fluorescent and photochemical data of wild-type phyA and Cph1 and their deletion mutants allowed localization of the site in the molecule responsible for the initial photochemical events to the N-terminal photosensory module (PSM) [42][44][45][46][42,44-46]. The site responsible for the phyA differentiation is localized in the NTE in the serine-rich cluster because the serine-to-alanine substitution of the first 10 serines (of rice phyA expressed in phyA-less Arabidopsis) resulted in the lack of the phyA′ pool with all the pigment present as phyA″ (Figure 4) [47]. Since phyA is a phosphoprotein the effect of this substitution suggested that the phyA modification could be serine phosphorylation. More specifically, (oat) phyA autophosphorylates at Ser8 and Ser18 (in the Pr and Pfr states) (see review [29]), and it was tempting to assume that this process may account for the phyA differentiation. The involvement of phosphorylation in the phyA differentiation is also pointed out by the fact that the lack of phytochrome kinase substrate 1 and 2 (PKS1 and PKS2) causes a shift in the phyA pools′ ratio toward phyA″ [48]. At the same time, there are data that contradict this concept. First, Ser8 and Ser18 are dispensable for the phyA″-into-phyA′ conversion. This is evidenced by the lack of the effect of the Ser8Ala and Ser18Ala substitutions on the phyA’/phyA″ balance [47]. Besides, the treatment of etiolated maize seedlings by okadaic (OA) and cantaridic (CA) acids (in stems) and by NaF (in roots) shifting the phosphatase/kinase equilibrium toward the former results in the increase in the phyA″ proportion, although one could expect to get more phyA′ at the expense of phyA″ (see [35][49][35,49] and discussion of this issue below). However, the option that some other serine(s) in the NTE serine cluster besides Ser8 and Ser18 is phosphorylated to form phyA′ from phyA″ is still open. Figure 4. Proportion of the two phytochrome pools, phyA′ and phyA″, in etiolated Arabidopsis plants of the different lines. Significantly different pairs of data (revealed by Fisher’s t-test for 6–12 independent measurements) are indicated: *, p < 0.05 for the same plant lines after brief (15 min) and prolonged (3 h) white light germination-inducing pre-treatment and **, p < 0.05 between different plant lines taken at the same pre-illumination time, 15 min or 3 h. From [47]. Irrespective of the concrete mechanism of the phyA diversification, it is clear that modifications of the NTE terminus greatly affect the photophysical and photochemical properties of the two phyA pools most likely through alterations in the conformation of the chromophore and its environment and changes in the strength and character of its interaction with the apoprotein. The activation barrier Ea critical for the Pr →lumi-R photoreaction should relate to the energy of the D-ring bonds fixing it in the chromophore pocket. During D-ring rotation these H bonds are to be broken (Figure 5) [42]. The energies associated with hydrogen bonds are in the range 6–30 kJ/mol [50] which fits well into the magnitudes of Ea in plant phytochromes (from hundreds J/mol to 30 kJ/mol depending on the phyA pools [7]) and Cph1 (3.0–6.5 and 12.0–17.5 kJ/mol for the two Cph1 species [42] (see above). Thus, the attribution of the Ea barrier to the H bonds of the D-ring fixing it in the chromophore pocket sounds quite reasonable. Figure 5. The chromophore D ring environment of Cph1 and its changes in the photoreaction. (a) 2VEA structure of Cph1 Pr [51] showing the interactions of the pyrrole N24 and C19 carboxyl oxygen; (b) hypothetical structure of prelumi-R at C15 = C16 isomerization angle of 117°. From [42] modified). Investigations of Cph1, as an adequate model of plant phytochrome allows for an explicit structure–function analysis of its molecule. Structural experiments reveal several critical points of interaction of the chromophore with its protein surrounding in Cph1. These are the C19 carbonyl oxygen of the D-ring forms a hydrogen bond with the nearby histidine His290 and a hydrogen bond between its pyrrole nitrogen N24 and a water molecule (Figure 5). The two highly conserved histidine residues, His323 and His372, are also involved in determining the conformation structure of the chromophore [52]. Of particular importance is the conserved tyrosine Tyr263 residue directly interacting with the D-ring [42][53][42,53]. Experiments with Cph1Δ2 and its mutants (Tyr263Phe, Tyr263His and Tyr263Ser) have shown that in contrast to the mutant Cph1Δ2 species, which are homogeneous, the WT Cph1Δ2 was found to be represented by two species. This is due to the structural plasticity of the chromophore provided by Tyr263. The two Cph1Δ2 species probably differ by the degree of their protonation [54, [54][55]55]. The second important tyrosine residue near the D-ring, which contributes to the structural inhomogeneity of Cph1, is Tyr176 [56] as revealed by ultrafast kinetic measurements of the photoprocesses in Cph1Δ in agreement with the static measurements of the plant phytochrome [37] and Cph1 [42][53][42,53]. The structural heterogeneity of the Pr state in Cph1 is also associated with the input of the hydrogen-bonding networks and charge distribution patterns around the chromophore as shown on Cph1 [52] and on (oat) phyA [57], and of multiple side chain conformations for several residues near the chromophore [51][53][51,53]. Thus, judging by the data on Cph1, the structure of the chromophore pocket in plant phytochrome provides freedom of movement removing sterical hindrances for the chromophore photoisomerization (Figure 5). This chromophore plasticity is reflected in the appearance of distinct conformers of the pigment—prevailing structures with energetically favourable chromophore-apoprotein interactions. The key role of the apoprotein matrix in determining the physicochemical phytochrome parameters is vividly observed when cyanobacterial phytochromes are compared with light-harvesting biliproteins [43]. In the latter, there is a rigid chromophore surrounding stiffly fixing the chromophore, reducing its freedom of torsional relaxation, and restricting its movements. This provides for the high fluorescence quantum yield of a pigment, a prerequisite for its being an efficient energy donor. Investigations of membrane-bound phytochrome date back to the early years of its research. Although the fact that the pigment was clearly shown to be soluble in in-vitro experiments, there were several publications reporting its pelletabily upon extraction and associations with different subcellular structures [58]]. In the experiments on maize coleoptiles [60] and Arabidopsis hypocotyls [61], it was shown that phyA″ possesses amphiphilic properties (an ambiquitous phyA type, i.e., having the ability to reversibly bind to subcellular structures [59])—it comprises both a water-soluble fraction and membrane-(protein-) associated fraction, in contrast to phyA′ which is only in the water-soluble state (Figure 6). Furthermore, phyA′ disappears upon deep dehydration of etiolated tissues [62] suggesting that phyA′ molecules need a water surrounding for their stability, and that in dry seeds phyA is likely to be present in the phyA′′ form. The properties of the presumably membrane- (protein-) associated phyA″ are close to those obtained in the sedimentation experiments in [63][64][63,64]. Figure 6. Proportion of phyA′ (light gray) and phyA″ (gray) in water-soluble and membrane-associated fractions of the intact 124 kDa maize phytochrome A in vitro. The error (SD) of the phyA content determination is ca ± 5%. From [60]. These changes in the hydrophobicity/hydrophilicity of phyA are likely to be the result of the overall structural rearrangement of the pigment upon its post-translational modification. One may hypothesize that the increased solubility of phyA′ is the result of the presumed phyA phosphorylation of serine(s) at the NTE terminus. The appearance in the molecule of a charged phosphoryl group(s) changes its overall conformation (as indicated by the drastic amendments of the energetics of the photoreaction) bringing about an increase in its polarity and, as a result, its hydrophilicity. Thus, there are three possible variants of the phyA state in the cell—the water-soluble phyA′ and the two fractions of amphiphilic phyA″, water-soluble and aggregated (protein-associated), which may explain the diverse and multiple functional phyA manifestations. In the process of light signal transduction, both phyA and phyB are imported into the nucleus in a light-dependent manner [8][9][8,9]. The phyA in its Pfr form needs association with plant-specific proteins FHY1 (Far-red elongated Hypocotyl 1) and FHL (FHY1-like) to achieve nuclear import [12][13][65][66][12,13,65,66]. The phyA amino-terminal extension (NTE) domain mediates the formation of the aggregates of phyA with its partners [67]. In the nucleus, phyA is localized to protein complexes known as photobodies (also speckles or spots); they are of two types—many small or a few large ones [8][65][68][8,65,68]. Of interest is the fact that phyA lacking the 6-12 amino acids from its N-terminus (Avena sativa (oat) Δ6–12 phyA-green fluorescent protein (phyA-GFP) expressed in Arabidopsis deficient in phyA) can form only one type of the complexes—many tiny spots [69]. It was shown that the WT phyA-GFP is represented in the cell by two phyAs—phyA′-GFP and phyA″-GFP. This implies that both of them are potential participants of the light-induced nuclear–cytoplasmic partitioning, and that the two types of light-induced phyA nuclear speckle formation may be connected with the existence of the two phyA species. This assumption is supported by the observation that Δ6–12 phyA-GFP, which forms only numerous tiny subnuclear speckles, is solely represented by phyA′-GFP [70]. The large speckles observed in the case of full-length phyA-GFP, besides the tiny speckles, may thus be associated with phyA″. Particular photoresponses in plants initiated by phytochromes are categorized into three photoresponse modes (for a review see [17][18][71][17,18,71]). These are the VLFR mode (observed under pulses of FR or very low fluences of pulsed or continuous R), the LFR mode (under the conditions of continuous or pulsed R), or the HIR mode (continuous FR). With the use of phyA and phyB Arabidopsis mutants, it was clearly shown that phyA mediates both the VLFR and HIR and phyB, the LFR [25]. A number of loci have been identified that affect differentially the VLFR and HIR [71]. A model is put forward in which phyA initiates two transduction pathways, the VLFR and HIR. The detection of the two phyAs implies, however, that the two modes of phyA responses, the VLFR and HIR, may differ also at the level of the effector, i.e., the distinct phyA species can separately initiate the different responses. Indeed, phyA mutants with substitutions or deletions at the NTE bringing about a steep decline or absence of one or the other phyA pool supports this hypothesis. It was shown that mutant rice phyA (phyA SA with the first 10 serines substituted by alanines) overexpressed in transgenic Arabidopsis deficient in phyA or both phyA and phyB comprises primarily or exclusively the phyA″ pool (see [47] and above). According to [26], these transgenic plants with the mutated rice phyA were much more active in the HIR responses than in the VLFR and LFR—in promoting under constant FR (1) inhibition of hypocotyl elongation, (2) anthocyanin accumulation, (3) agravitropic growth, and (4) ‘FR-killing effect’ (lethality of seedlings grown under FR upon illumination with R or W light [73]). This indicates that phyA″ is primarily responsible for the HIR of these deetiolation processes. The phyAʹʹ was also found to be much more effective in germination induction than phyA′ [47]. In contrast, WT rice phyA, which was represented by both phyA′ and phyA″, was more active in (1) inhibition of hypocotyl elongation and cotyledon opening under pulses of FR light (VLFR), (2) ‘FR killing effect’ after FR-light pulses (VLFR), (3) inhibition of hypocotyl elongation and agravitropic responses under R (LFR). This VLFR activity may thus be connected with the presence of phyA′ since phyA SA comprising phyA″ revealed itself as a mediator of primarily the HIR (Figure 7). The phyA″ is a more appropriate candidate for the LFR activity than the phyA′ because it is closer by its properties to phyB responsible for the LFR (photochemically and by light-stability; see above) than phyA′. This attribution of the response modes is supported by experiments with Figure 7. Working scheme of the state and functions of the native phytochrome A pools in etiolated seedlings. De novo synthesized phyA in germinating seeds and growing seedlings is initially in the phyA′′ form, which possesses amphiphilic properties and is present in the cell in water-soluble and membrane– (protein–) associated (phyA′′m) fractions. In darkness, phyA′′ is converted into the water-soluble phyA′ form, possibly, via serine phosphorylation at the N-terminus of the molecule. Upon illumination, the water-soluble phyA′ and phyA′′ are transported into the nucleus forming two different types of nuclear speckles and inducing different modes of photoresponses, the VLFRs and HIRs, respectively. phyA′′m in the Pfr form remains in the cytoplasm and initiates regulation processes there. Pointed arrows indicate the stimulation effects; blunt-ended arrow, the inhibitory effects. From [74]. the truncated Δ6–12 oat phyA expressed in transgenic tobacco and Arabidopsis, which is represented primarily by phyA′ (see above and [70]). Casal et al. [69] have shown that this truncated phyA was as active as the full-length phyA for the VLFR of hypocotyl growth inhibition, cotyledon unfolding and blocking subsequent greening under white light in Arabidopsis. In transgenic tobacco, it was hyperactive in the VLFR of hypocotyl growth inhibition and cotyledon unfolding. In both the plant species, Δ6–12 oat phyA revealed a dominant suppression of the HIR in these regulation reactions. These data suggest that in these expression systems the VLFR are mediated by phyA′ (see Figure 7) and [74]). The fact that phyA’ and phyA” form different types of speckles in the nucleus (see [70] and above) suggests that the distinct modes of photoreposponses they mediate (the VLFR and HIR, respectively) proceed via different signal transduction chains in agreement with [71][72][71,72]. Besides the above phyA mutants with the complete block of the VLFR or HIR, there are modifications in the phyA molecule increasing or decreasing the strength of these responses. Of particular interest are those which relate to the natural modifications of phyA (autophosphorylation) or to its mechanism of action (kinase activity)—phyA is known as a phosphoprotein and a light-regulated kinase [77][78][77,78]. The photoreceptor is autophosphorylated at serines 8 and 18 (in oats), and this serves as a means regulation its functional activity [79][80][81][79–8 1]. Mutations at Ser8Ala and Ser18Ala in oat phyA expressed in Arabidopsis, at the sites involved in the phyA autophosphorylation bring about hypersensitivity to FRc and FRp, which is interpreted to result from the higher light stability of the mutated phyA [81]. There were no significant changes in the phyA′ / phyA″ ratio in this Arabidopsis line, suggesting that the effect is not connected with changes in their content but, rather, in their higher stability [82]. The character of the photoregulation reactions of the distinct phyA species may not be strictly fixed and is susceptible to the physiological context. The strength of the phyAs photoresponses, their sign and their mode depend on plant species and genotype, their age, and organ/tissue used. The phyA′ acquires the properties of phyA″—higher stability and the HIR mode—in overexpressors of phyA (primarily in the phyA′ state) transgenic wheat [75] and potato [76]. There is down-regulation (under the conditions of the HIR) of the active protochlorophyllide (Pchlide655) accumulation in the cotyledons of dicot tomato and Arabidopsis [83] and monocot rice and its mutants deficient in phyA, phyB, or phyA and phyB [41][41] . However, in tobacco cotyledons and pea leaves, and in stems of tobacco, pea, tomato, and Arabidopsis, a positive effect was observed [83]. In more detail, see a discussion on phyA regulation of Pchlide biosynthesis in [84]. Finally, the hormone jasmonic acid (JA) on the phyAs and their functions [85,86]. JA controls different aspects of plant growth and development, including inhibition of seed germination and root growth and stimulation of degradation of chloroplast proteins and leaf senescence. Experiments with rice mutants (hebiba and cpm2) lacking JA have clearly shown that JA determines the sign of the phyA regulatory effects. JA reduces the phyA functional activity primarily in its phyA′′ form mediating the HIR (see the discussion on the JA and phyA interaction in [74][86][74,86]). A number of early research indicate that there are biophysical events initiated by phytochrome in etiolated plant cells, such as modulation of ion flux and electric potential across plasma membranes. Direct experimental evidence that phyA acts in the cytoplasm was obtained with the use of mutants with a blocked process of nuclear-cytoplasmic phyA partitioning. Certain photophysiological responses (such as R-enhanced phototropism, abrogation of gravitropism, and inhibition of hypocotyl elongation in blue light) are seen in the mutants lacking FHL and FHY1, which are necessary for the appearance of the nuclear-localized fraction of phyA after its light activation [12][13][14][15][16][12–16]. The integration between the phytochrome A and phototropin regulatory pathways involves Phytochrome Kinase Substrate 1 (PKS1)— a protein associated with the plasma membrane, which interact with both PHYA and PHOT1 [87][88][87,88]. The effects of phytochrome on hypocotyl growth involve an integral membrane-bound protein associated with auxin transport [89]. The control of translation of PORA mRNA is also mediated by cytoplasmic phyA [15][16][15,16]. The phyA effects are likely to be realized through direct interaction of the photoreceptor with plasma membrane protein partners. Ion fluxes across the plasma membrane may participate in light-invoked signal transduction [90][190]. Recent findings widen the range of cytosolic regulatory events. It was shown that modification of transcriptional processes may also take place in the cytoplasm [15][91][15,91]. Phytochrome participates in regulating phototropic responses and primary root elongation growth [92][93][94][92–94]. Cytoplasmic phytochrome action is observed in root development [94]. Given that the association of phyA with cytosolic proteins, in particular, with PKS1, is required for the above cytosolic photoregulation effects, One may assume that the most likely candidate for this association is amphiphilic phyA″. Its affinity for protein association makes phyA″ (its membrane-associated fraction phyA′′m) the most likely candidate for these functions. The other fraction of phyA″, which is not bound to the membrane (protein), is involved together with the whole pool of the soluble phyA′ in the nuclear regulation events. In this connection, it is interesting to note that PKS1 and PKS2 are intimately related to the state of the phyA pools in the cell [48]. It was found that the Arabidopsis pks1pks2 double mutant has a much greater proportion of phyA″ at the expense of phyA′ . It is tempting to hypothesize that the phyA′′m pool could be also responsible for the fast photoregulation effects in the cytoplasm, such as modulation of ion transport, electric potentials, and cytoplasm fluidity (Figure 7). Thus, the difference in the hydrophilicity/hydrophobicity of the two phyA pools (see above) may account for the specificity of phyA functioning in the cytoplasm. Light has specific impacts on different plant tissues and organs during the process of photomorphogenesis and throughout various stages of the plant life cycle—promoting growth in some of them and inhibiting expansion in others. The detection of the two phyA populations makes the functional behaviour of the photoreceptor even more complicated. It has been firmly documented that the content of the two phyAs and their ratio are strongly dependent on the plant species, their organs and tissues used and stage of the plant’s development. In general, phyA′ is a labile species dominating in growing etiolated tissues, whereas phyA″ is more stable with a high proportion in resting tissues [31][34][75][31,34,75]. The mechanisms determining this complex behaviour of phyAs remain unclear, although there are indications that the phosphatase/kinase equilibrium in the cell and cytoplasmic pH affect it [35][49][35,49]. The agents suppressing phosphatase activity and shifting the phosphatase/kinase equilibrium towards kinases (okadaic and cantaridic acids—inhibitors of phosphatases of the PP1 and PP2A types, and NaF—phosphatase inhibitor of a broad spectrum) brought about the increase in the phyA″ content relative to that of phyA′ due to the elevated destruction of the latter or its conversion into phyA″. The phyA’/phyA″ ratio was found to be connected with pH. These effects are intimately connected with the regulation of the complex processes of phyAs biosynthesis, interconversion and destruction. Even more pronounced effects on the phyA pools are seen after pre-illumination. They are clearly distinguished into those induced by red and far-red light, which may reflect the specificity of the phyA action as a mediator of the far-red light: fast destruction (tens of minutes) of phyA′ [40] under R and a total phyA decline of both the phyA pools in seedlings grown under FR [96]. Judging by the fact that the de-etiolated lip mutant (of pea) without pre-illumination reveals similar properties as the FR pre-illuminated WT pea, i.e., total phyA decline without phyAs ratio shift [97], one may conclude that this FR effect is primarily a consequence of the negative feedback of the phyA autoregulation of its own synthesis during de-etiolation. Of interest is the delayed effect of germination-inducing R pre-illumination of Arabidopsis seeds on the state of the two phyAs in growing seedlings [47]. It was stimulating for the formation of phyA′ as compared with the seedlings without such a light pretreatment. This suggests that, besides the phyA′ destruction, R light may stimulate phyA′ formation, possibly modulating seedling’s development, and that the phyA differentiation into the two subspecies is a light-regulated process. Collectively, these data suggest that it is important to consider the variations in the content and proportion of the phyA pools as a part of the very complex process of phyA fine-tuning. See in more detail the discussion on all the aspects of the problem of the two phyA types in plants in [98]. Detection of phyA fluorescence in-situ and the development of the sensitive fluorescence method made it possible to assay phyA in detail in its native state in the cell. The pigment characteristics—fluorescence emission and excitation (absorption) spectra, activation and kinetic parameters of the photoreaction, and content in tissues—greatly varied depending on plant species/organ, their developmental state, and the effect of environmental factors. This was interpreted as the existence of two phyA states which appear as a result of post-translational modification, possibly, phosphorylation—the first is the major, light-labile, photochemically active at Tc and hydrophilic (phyA’), and the second, the minor, saturable by its content, relatively light-stable, inactive at Tc and amphiphilic (phyA″). Both of them are the full-lenth products of the same PHYA gene and reveal normal photochemical activity at Ta. The phyA″ is present in the cell in a water-solved state and in association with the membrane (protein) (designated phyA″m). Their photochemical differences are explained in terms of concrete chromophore-apoprotein interactions. The three states of phyA are believed to be responsible for the complex phyA action: phyA′ and phyA″ were shown to differ by the mode of the nuclear-cytoplasmic partitioning and mediate the VLFR and the HIR, respectively. The phyA″m is hypothesized to participate in the cytoplasmic photoregulation effects. The character of the phyAs photoresponses—their mode, strength, and sign—depend on the plant (wild type, phytochrome mutants and overexpressors, mutants lacking the hormone jasmonic acid) and organ/tissue. The concrete mechanism of the phyA differentiation needs, however, clarification. 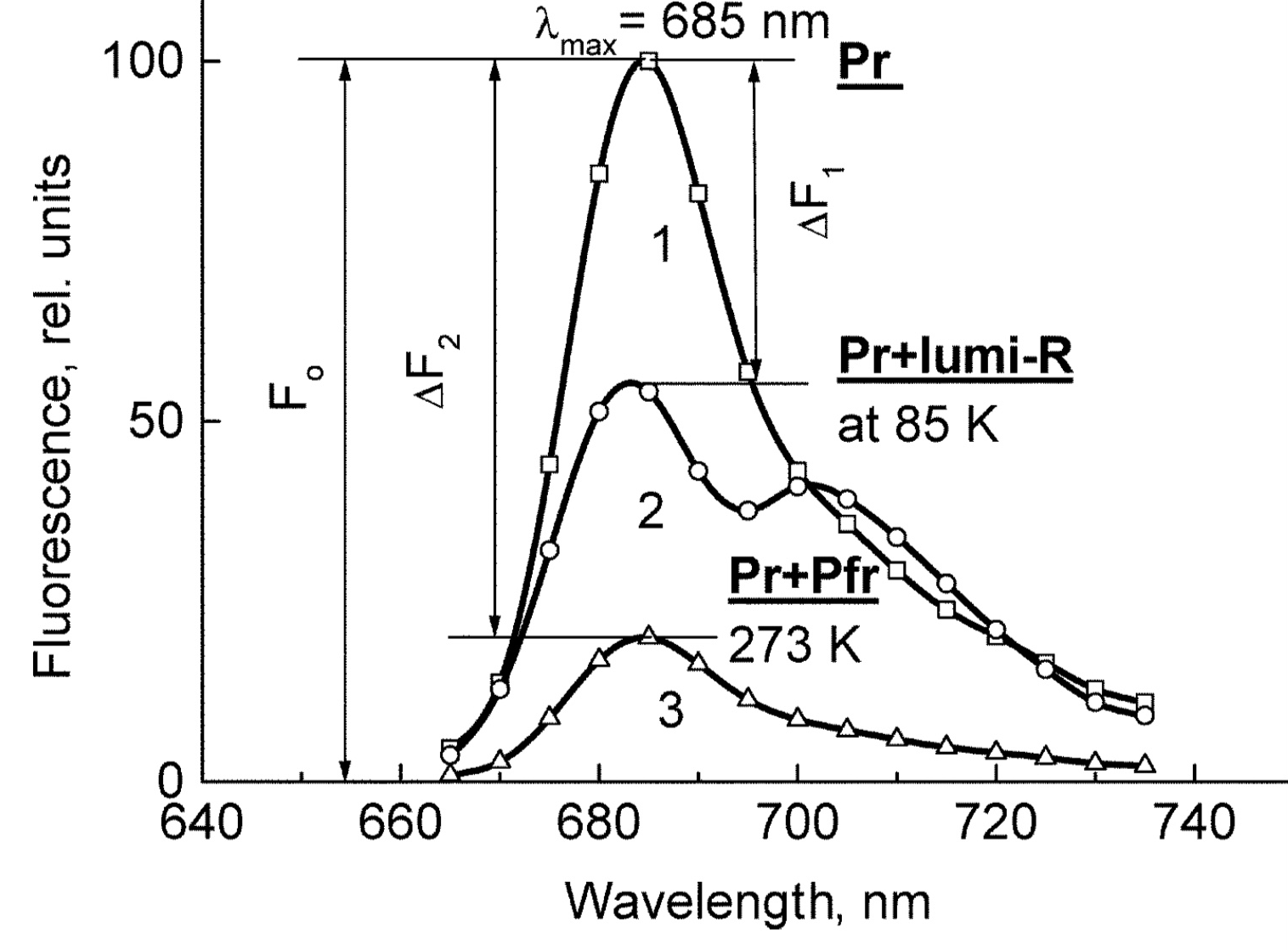
32. Photochemical and Structural Characterization of the Two phyA Types
4. The phyA Pools and the Problem of the Membrane- (Protein-) Association of Phytochrome
5. The Two phyA Spices May Account for the Two Distinct Patterns of phyA Nuclear Speckle Formation
6. The phyAs Mediate Distinct Types of Photoresponses: The Major and Light-Labile phyA′—The VLFR, and the Minor and Relatively Light-Stable phyA″—The HIR
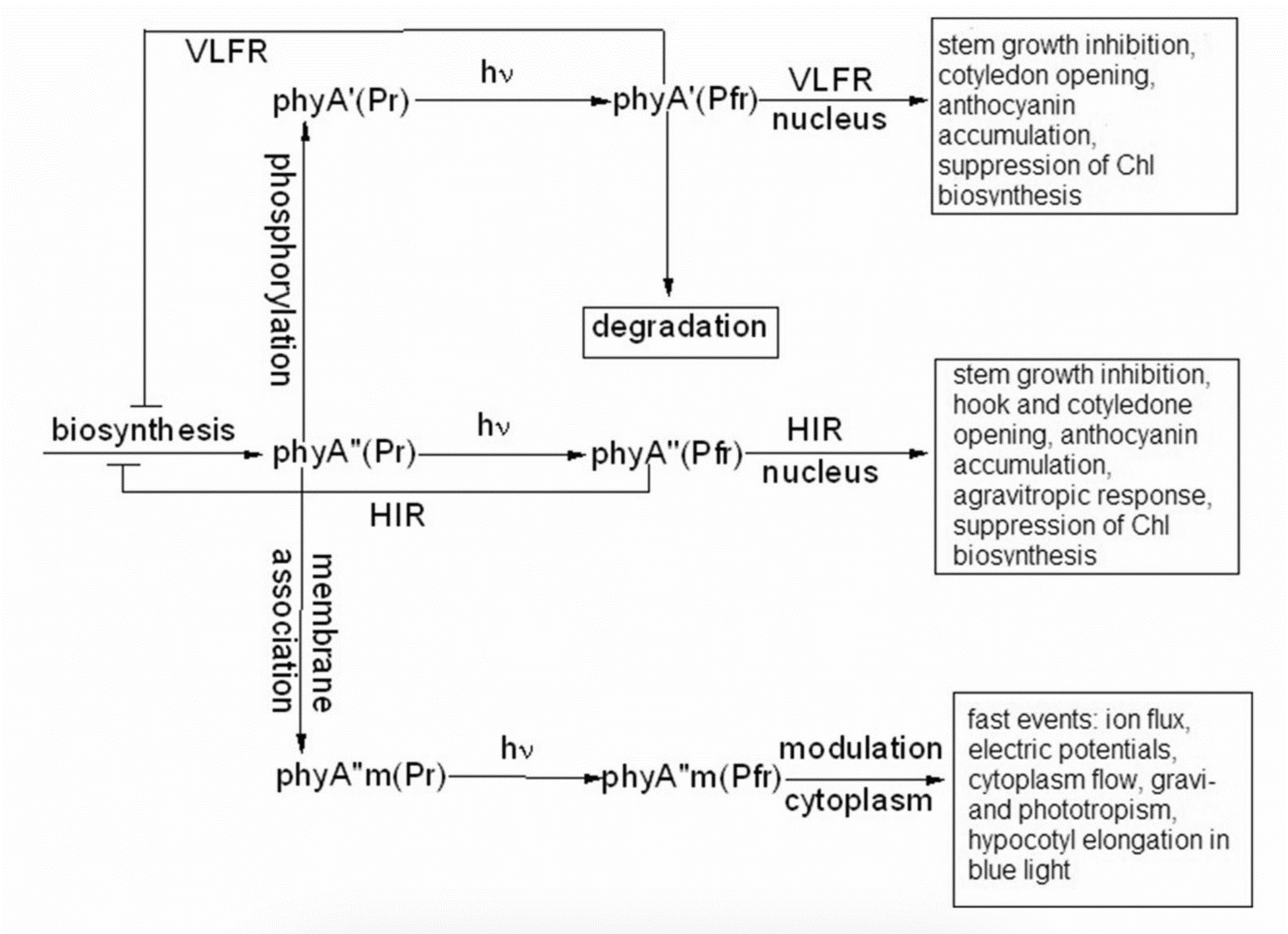
7. Action of phyA in the Cytoplasm
8. Regulation of the phyAs Content and Their Balance in the Dark and in the Light
9. Conclusions
References
- Smith, H. Photomorphogenesis. Plant Cell Environ. 1997, 20, 657–844.
- Inoue, K.; Nishihama, R.; Kohchi, T. Evolutionary origin of phytochrome responses and signaling in land plants. Plant Cell Environ. 2017, 40, 2502–2508.
- Zakurin, A.O.; Shchennikova, A.V.; Kamionskaya, A.M. Artificial-light culture in protected ground plant growing: Photosynthesis, photomorphogenesis, and prospects of LED application. Russ. J. Plant Physiol. 2020, 67, 413–424.
- Chernov, K.G.; Redchuk, T.A.; Omelina, E.S.; Verkhusha, V.V. Near-infrared fluorescent proteins, biosensors, and optogenetic tools engineered from phytochromes. Chem. Rev. 2017, 117, 6423–6446.
- Furuya, M. History and insights. In Light Sensing in Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 3–18.
- Braslavsky, S.E.; Gärtner, W.; Schaffner, K. Phytochrome photoconversion. Plant Cell Environ. 1997, 20, 700–706.
- Sineshchekov, V.A. Photobiophysics and photobiochemistry of the heterogeneous phytochrome system. Biochim. Biophys. Acta (BBA)-Bioenerg. 1995, 1228, 125–164.
- Kircher, S.; Kozma-Bognar, L.; Kim, L.; Adam, E.; Harter, K.; Schäfer, E.; Nagy, F. Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 1999, 11, 1445–1456.
- Hisada, A.; Hanzawa, H.; Weller, J.L.; Nagatani, A.; Reid, J.B.; Furuya, M. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 2000, 12, 1063–1078.
- Legris, M.; Ince, Y.C.; Fankhauser, C. Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun. 2019, 10, 5219.
- Cheng, M.C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244.
- Hiltbrunner, A.; Tscheuschler, A.; Viczian, A.; Kunkel, T.; Kircher, S.; Schäfer, E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034.
- Rösler, J.; Klein, I.; Zeidler, M. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 2007, 104, 10737–10742.
- Jaedicke, K.; Lichtenthäler, A.L.; Meyberg, R.; Zeidler, M.; Hughes, J. A phytochrome–phototropin light signaling complex at the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 12231–12236.
- Paik, I.; Yang, S.; Choi, G. Phytochrome regulates translation of mRNA in the cytosol. Proc. Natl. Acad. Sci. USA 2012, 109, 1335–1340.
- Hughes, J. Phytochrome cytoplasmic signaling. Annu. Rev. Plant Biol. 2013, 64, 377–402.
- Casal, J.J.; Sanchez, R.A.; Yanovsky, M.J. The function of phytochrome A. Plant Cell Environ. 1997, 20, 813–819.
- Casal, J.J.; Luccioni, L.G.; Oliverio, K.A.; Boccalandro, H.E. Light, phytochrome signalling and photomorphogenesis in Arabidopsis. Photochem. Photobiol. Sci. 2003, 2, 625–636.
- Brockmann, J.; Schaufer, E. Analysis of Pfr destruction in Amaranthus caudatus L Evidence for two pools of phytochrome. Photochem. Photobiol. 1982, 35, 555–558.
- Tokuhisa, J.G.; Daniels, S.M.; Quail, P.H. Phytochrome in green tissue: Spectral and immunochemical evidence for two distinct molecular species of phytochrome in light-grown Avena sativa L. Planta 1985, 164, 321–332.
- Sineshchekov, V.A.; Sineshchekov, A.V. Fluorescence of phytochrome in the cells of etiolated pea seedlings. Biophysics 1987, 32, 116–122.
- Sineshchekov, V.A.; Sineshchekov, A.V. Fluorescence of phytochrome in the cells of dark‐grown plants and its connection with the phototransformations of the pigment. Photochem. Photobiol. 1989, 49, 325–330.
- Sharrock, R. A.; Quail, P. H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes & development, 1989, 3(11), 1745-1757.
- Clack, T.; Mathews, S.; Sharrock, R.A. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 1994, 25, 413–427.
- Yanovsky, M.; Casal, J.; Luppi, J. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very‐low‐fluence and high‐irradiance responses. Plant J. 1997, 12, 659–667.
- Kneissl, J.; Shinomura, T.; Furuya, M.; Bolle, C. A rice phytochrome A in Arabidopsis: The role of the N-terminus under red and far-red light. Mol. Plant 2008, 1, 84–102.
- Long, C.; Iino, M. Light-dependent osmoregulation in pea stem protoplasts. Photoreceptors, tissue specificity, ion relationships, and physiological implications. Plant Physiol. 2001, 125, 1854–1869.
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038.
- Choi, D.M.; Kim, S.H.; Han, Y.J.; Kim, J.I. Regulation of Plant Photoresponses by Protein Kinase Activity of Phytochrome A. Int. J. Mol. Sci. 2023, 24, 2110.
- Butler, W.L.; Norris, K.H.; Siegelman, H.W.; Hendricks, S.B. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1703–1708.
- Sineshchekov, V. Two spectroscopically and photochemically distinguishable phytochromes in etiolated seedlings of monocots and dicots. Photochem. Photobiol. 1994, 59, 77–85.
- Sineshchekov, V.A. Phytochrome A: Functional diversity and polymorphism. Photochem. Photobiol. Sci. 2004, 3, 596–607.
- Sineshchekov, V.A.; Sineshchekov, A.V. Different photoactive states of the red phytochrome form in the cells of etiolated pea and oat seedlings. J. Photochem. Photobiol. B Biol. 1990, 5, 197–217.
- Sineshchekov, V.; Ogorodnikova, O.; Thiele, A.; Gatz, C. Fluorescence and photochemical characterization of phytochromes A and B in transgenic potato expressing Arabidopsis phytochrome B. J. Photochem. Photobiol. B Biol. 2000, 59, 139–146.
- Sineshchekov, V.; Koppel, L.; Shor, E.; Kochetova, G.; Galland, P.; Zeidler, M. Protein phosphatase activity and acidic/alkaline balance as factors regulating the state of phytochrome A and its two native pools in the plant cell. Photochem. Photobiol. 2013, 89, 83–96.
- Sineshchekov, V.; Koppel, L.; Okamoto, H.; Wada, M. Fern Adiantum capillus-veneris phytochrome 1 comprises two native photochemical types similar to seed plant phytochrome A. J. Photochem. Photobiol. B Biol. 2014, 130, 20–29.
- Sineshchekov, V.A.; Akhobadze, V.V. Phytochrome states in etiolated pea seedlings: Fluorescence and primary photoreactions at low temperatures. Photochem. Photobiol. 1992, 56, 743–749.
- Sineshchekov, V.A. Evidence for the existence of two phytochrome A populations. J. Photochem. Photobiol. B Biol. 1995, 28, 53–55.
- Sineshchekov, V.A.; Ogorodnikova, O.B.; Devlin, P.F.; Whitelam, G.C. Fluorescence spectroscopy and photochemistry of phytochromes A and B in wild-type, mutant and transgenic strains of Arabidopsis thaliana. J. Photochem. Photobiol. B Biol. 1998, 42, 133–142.
- Sineshchekov, V.A.; Ogorodnikova, O.B.; Weller, J.L. Fluorescence and photochemical properties of phytochromes A and B in etiolated pea seedlings. J. Photochem. Photobiol. B Biol. 1999, 49, 204–211.
- Sineshchekov, V.; Loskovich, A.; Inagaki; N.; Takano, M. Two native pools of phytochrome A in monocots: Evidence from fluorescence investigations of phytochrome mutants of rice. Photochem. Photobiol. 2006, 82, 1116–1122.
- Sineshchekov, V.; Mailliet, J.; Psakis, G.; Feilke, K.; Kopycki, J.; Zeidler, M.; Essen, L.-O.; Hughes, J. Tyrosine 263 in cyanobacterial phytochrome Cph1 optimizes photochemistry at the prelumi-R→lumi-R step. Photochem. Photobiol. 2014, 90, 786–795.
- Sineshchekov, V.A.; Bekasova, O.D. Two distinct photoprocesses in cyanobacterial bilin pigments: Energy migration in light‐harvesting phycobiliproteins versus photoisomerization in phytochromes. Photochem. Photobiol. 2020, 96, 750–767.
- Sineshchekov, V.A.; Clough, R.C.; Jordan‐Beebe, E.T.; Vierstra, R.D. Fluorescence analysis of oat phyA deletion mutants expressed in tobacco suggests that the N‐terminal domain determines the photochemical and spectroscopic distinctions between phyA′ and phyA″. Photochem. Photobiol. 1999, 69, 728–732.
- Sineshchekov, V.; Koppel’, L.; Esteban, B.; Hughes, J.; Lamparter, T. Fluorescence investigation of the recombinant cyanobacterial phytochrome (Cph1) and its C-terminally truncated monomeric species (Cph1Δ2): Implication for holoprotein assembly, chromophore-apoprotein interaction and photochemistry. J. Photochem. Photobiol. B Biol. 2002, 67, 39–50.
- Sineshchekov, V.; Hennig, L.; Lamparter, T.; Hughes, J.; Gärtner, W.; Schäfer, E. Recombinant phytochrome A in yeast differs by its spectroscopic and photochemical properties from the major phyA′ and is close to the minor phyA″: Evidence for posttranslational modification of the pigment in plants. Photochem. Photobiol. 2001, 73, 692–696.
- Sineshchekov, V.A.; Koppel, L.A.; Bolle, C. Two native types of phytochrome A, phyA′ and phyA″, differ by the state of phosphorylation at the N-terminus as revealed by fluorescence investigations of the Ser/Ala mutant of rice phyA expressed in transgenic Arabidopsis. Funct. Plant Biol. 2016, 45, 150–159.
- Sineshchekov, V.; Fankhauser, C. PKS1 and PKS2 affect the phyA state in etiolated Arabidopsis seedlings. Photochem. Photobiol. Sci. 2004, 3, 608–611.
- Sineshchekov, V.; Shor, E.; Koppel, L. The phosphatase/kinase balance affects phytochrome A and its native pools, phyA′ and phyA″, in etiolated maize roots: Evidence from the induction of phyA′ destruction by a protein phosphatase inhibitor sodium fluoride. Photochem. Photobiol. Sci. 2021, 20, 1429–1437.
- Haynie, D.T. Biological Thermodynamics; Cambridge University Press: Cambridge, UK, 2001.
- Essen, L.-O.; Mailliet, J.; Hughes, J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. USA 2008, 105, 14709–14714.
- Song, C.; Essen, L.O.; Gärtner, W.; Hughes, J.; Matysik, J. Solid-state NMR spectroscopic study of chromophore-protein interactions in the Pr ground state of plant phytochrome A. Mol. Plant. 2012, 5, 698–715.
- Mailliet, J.; Psakis, G.; Feilke, K.; Sineshchekov, V.; Essen, L.O.; Hughes, J. Spectroscopy and a high-resolution crystal structure of Tyr263 mutants of cyanobacterial phytochrome Cph1. J. Mol. Biol. 2011, 413, 115–127.
- Rumfeldt, J.A.; Takala, H.; Liukkonen, A.; Ihalainen, J.A. UV‐vis spectroscopy reveals a correlation between Y263 and BV protonation states in bacteriophytochromes. Photochem. Photobiol. 2019, 95, 969–979.
- Kirpich, J.S.; Mix, L.T.; Martin, S.S.; Rockwell, N.C.; Lagarias, J.C.; Larsen, D.S. Protonation heterogeneity modulates the ultrafast photocycle initiation dynamics of phytochrome Cph1. J. Phys. Chem. Lett. 2018, 9, 3454–3462.
- Kim, P.W.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Larsen, D.S. Dynamic inhomogeneity in the photodynamics of cyanobacterial phytochrome Cph1. Biochemistry 2014, 53, 2818–2826.
- Song, C.; Psakis, G.; Lang, C.; Mailliet, J.; Gärtner, W.; Hughes, J.; Matysik, J. Two ground state isoforms and a chromophore D-ring photoflip triggering extensive intramolecular changes in a canonical phytochrome. Proc. Natl. Acad. Sci. USA 2011, 108, 3842–3847.
- Roux, S.J. Phytochrome and membranes. In Photomorphogenesis in Plants; Springer: Berlin/Heidelberg, Germany, 1986; pp. 115–134.
- Clegg, J.S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 246, R133–R151.
- Sineshchekov, V.; Lamparter; T.; Hartmann, E. Evidence for the existence of membrane‐associated phytochrome in the cell. Photochem. Photobiol. 1994, 60, 516–520.
- Sineshchekov, V.A. (MV Lomonosov Moscow State University, Moscow, Russia); Zeidler, M. (Justus-Liebig-Universität, Gießen, German). Unpublished results, 2012.
- Sineshchekov, V.A. Extreme dehydration of plant tissues irreversibly converts the major and variable phyA′ into the minor and conserved phyA″. J. Photochem. Photobiol. B Biol. 2006, 85, 85–91.
- Lamparter, T.; Lutterbuese, P.; Schneider‐Poetsch, H.A.W.; Hertel, R. A study of membrane‐associated phytochrome: Hydrophobicity test and native size determination. Photochem. Photobiol. 1992, 56, 697–707.
- Terry, M.J.; Hall, J.L.; Thomas, B. The association of type I phytochrome with wheat leaf plasma membranes. J. Plant Physiol. 1992, 140, 691–698.
- Kim, L.; Kircher, S.; Toth, R.; Adam, E.; Schäfer, E.; Nagy, F. Light‐induced nuclear import of phytochrome‐A: GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 2000, 22, 125–133.
- Zhou, Q.; Hare, P.D.; Yang, S.W.; Zeidler, M.; Huang, L.F.; Chua, N.H. FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 2005, 43, 356–370.
- .Sokolova, V.; Bindics, J.; Kircher, S.; Ádám, É.; Schäfer, E.; Nagy, F.; Viczián, A. Missense mutation in the amino terminus of phytochrome A disrupts the nuclear import of the photoreceptor. Plant Physiol. 2012, 158, 107–118.
- Van Buskirk, E.K.; Decker, P.V.; Chen, M. Photobodies in light signaling. Plant Physiol. 2012, 158, 52–60.
- Casal, J.J.; Davis, S.J.; Kirchenbauer, D.; Viczian, A.; Yanovsky, M.J.; Clough, R.C.; Vierstra, R.D. The serine-rich N-terminal domain of oat phytochrome A helps regulate light responses and subnuclear localization of the photoreceptor. Plant Physiol. 2002, 129, 1127–1137.
- Sineshchekov, V.; Sudnitsin, A.; Ádám, É.; Schäfer, E.; Viczián, A. phyA-GFP is spectroscopically and photochemically similar to phyA and comprises both its native types, phyA’ and phyA″. Photochem. Photobiol. Sci. 2014, 13, 1671–1679.
- Casal, J.J.; Sánchez, R.A.; Botto, J.F. Modes of action of phytochromes. J. Exp. Bot. 1998, 49, 127–138.
- Cerdán, P.D.; Staneloni, R.J.; Ortega, J.; Bunge, M.M.; Rodriguez-Batiller, M.J.; Sánchez, R.A.; Casal, J.J. Sustained but not transient phytochrome A signaling targets a region of an Lhcb1* 2 promoter not necessary for phytochrome B action. Plant Cell 2000, 12, 1203–1211.
- Barnes, S.A.; Nishizawa, N.K.; Quaggio, R.B.; Whitelam, G.C.; Chua, N.H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell 1996, 8, 601–615.
- Sineshchekov, V.; Koppel, L. Phytochrome A in plants comprises two structurally and functionally distinct populations—Water-soluble phyA′ and amphiphilic phyA″. Biophys. Rev. 2022, 14, 905–921.
- Sineshchekov, V.; Koppel, L.; Shlumukov, L.; Barro, F.; Barcelo, P.; Lazzeri, P.; Smith, H. Fluorescence and photochemical properties of phytochromes in wild‐type wheat and a transgenic line overexpressing an oat phytochrome A (PHYA) gene: Functional implications. Plant Cell Environ. 2001, 24, 1289–1297.
- Sineshchekov, V.A.; Heyer, A.G.; Gatz, C. Phytochrome states in transgenic potato plants with altered phytochrome A levels. J. Photochem. Photobiol. B Biol. 1996, 34, 137–142. https://doi.org/10.1016/ 1011-134407284-3.
- Yeh, K.C.; Lagarias, J.C. Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 1998, 95, 13976–13981.
- McMichael, R.W., Jr.; Lagarias, J.C. Phosphopeptide mapping of Avena phytochrome phosphorylated by protein kinases in vitro. Biochemistry 1990, 29, 3872–3878.
- Lapko, V.N.; Jiang, X.Y.; Smith, D.L.; Song, P.S. Mass spectrometric characterization of oat phytochrome A: Isoforms and posttranslational modifications. Protein Sci. 1999, 8, 1032–1044.
- Kim, J.I.; Park, J.E.; Zarate, X.; Song, P.S. Phytochrome phosphorylation in plant light signaling. Photochem. Photobiol. Sci. 2005, 4, 681–687.
- Han, Y.J.; Kim, H.S.; Kim, Y.M.; Shin, A.Y.; Lee, S.S.; Bhoo, S.H.; Kim, J.I. Functional characterization of phytochrome autophosphorylation in plant light signaling. Plant Cell Physiol. 2010, 51, 596–609.
- Sineshchekov, V.; Koppel, L.; Kim, J.I. The dephosphorylated S8A and S18A mutants of (oat) phytochrome A comprise its two species, phyA’ and phyA’’, suggesting that autophosphorylation at these sites is not involved in the phyA differentiation. Photochem. Photobiol. Sci. 2019, 18, 1242–1248.
- Sineshchekov, V.; Belyaeva, O.; Sudnitsin, A. Up-regulation by phytochrome A of the active protochlorophyllide, Pchlide655, biosynthesis in dicots under far-red light. J. Photochem. Photobiol. B Biol. 2004, 74, 47–54.
- Sineshchekov, V.A.; Belyaeva, O.B. Regulation of chlorophyll biogenesis by phytochrome A. Biochemistry 2019, 84, 491–508.
- Sineshchekov, V.A.; Loskovich, A.V.; Riemann, M.; Nick, P. The jasmonate-free rice mutant hebiba is affected in the response of phyA′/phyA″ pools and protochlorophyllide biosynthesis to far-red light. Photochem. Photobiol. Sci. 2004, 3, 1058–1062.
- Sineshchekov, V.; Koppel, L.; Riemann, M.; Nick, P. Phytochrome A and its Functional Manifestations in Etiolated and Far‐red Light‐grown Seedlings of the Wild‐type Rice and its Hebiba and Cpm2 Mutants Deficient in the Defense‐related Phytohormone Jasmonic Acid. Photochem. Photobiol. 2021, 97, 335–342.
- Fankhauser, C.; Yeh, K.C.; Lagarias, J.C.; Zhang, H.; Elich, T.D.; Chory, J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 1999, 284, 1539–1541.
- Lariguet, P.; Schepens, I.; Hodgson, D.; Pedmale, U.V.; Trevisan, M.; Kami, C.; Fankhauser, C. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 2006, 103, 10134–10139.
- Titapiwatanakun, B.; Murphy, A.S. Post-transcriptional regulation of auxin transport proteins: Cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. J. Exp. Bot. 2009, 60, 1093–1107.
- Kim, K.B.; Park, M.H.; Chae, Q. Light effects on the membrane potential in oat cells. BMB Rep. 1995, 28, 382–386.
- Schwenk, P.; Hiltbrunner, A. Phytochrome A mediates the disassembly of processing bodies in far-red light. Front. Plant Sci. 2022, 13, 828529.
- Correll, M.J.; Kiss, J.Z. The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol. 2005, 46, 317–323.
- Correll, M.J.; Coveney, K.M.; Raines, S.V.; Mullen, J.L.; Hangarter, R.P.; Kiss, J.Z. Phytochromes play a role in phototropism and gravitropism in Arabidopsis roots. Adv. Space Res. 2003, 31, 2203–2210.
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438.
- Shin, D.H.; Cho, M.H.; Kim, T.L.; Yoo, J.; Kim, J.I.; Han, Y.J.; Hahn, T.R. A small GTPase activator protein interacts with cytoplasmic phytochromes in regulating root development. J. Biol. Chem. 2010, 285, 32151–32159.
- Sineshchekov, V.A.; Weller, J.L. Two modes of the light-induced phytochrome A decline–with and without changes in the proportion of its isoforms (phyA′ and phyA″): Evidence from fluorescence investigations of mutant phyA-3D pea. J. Photochem. Photobiol. B Biol. 2004, 75, 127–135.
- Sineshchekov, V.A.; Frances, S.; White, M.J. Fluorescence and photochemical characterization of phytochrome in de-etiolated pea mutant lip. J. Photochem. Photobiol. B Biol. 1995, 28, 47–51.
- Sineshchekov, V.A. Two distinct molecular types of phytochrome A in plants: evidence of existence and implications for functioning. Int. J. Mol. Sci., 2023, 24(9), 8139.
