Immunotherapy has remarkably revolutionized the management of advanced hepatocellular carcinoma (HCC) and prompted clinical trials, with therapeutic agents being used to selectively target immune cells rather than cancer cells. Currently, tThere is great interest in the possibility of combining locoregional treatments with immunotherapy for HCC, as this combination is emerging as an effective and synergistic tool for enhancing immunity. On the one hand, immunotherapy could amplify and prolong the antitumoral immune response of locoregional treatments, improving patients’ outcomes and reducing recurrence rates. On the other hand, locoregional therapies have been shown to positively alter the tumor immune microenvironment and could therefore enhance the efficacy of immunotherapy.
- hepatocellular carcinoma
- immunotherapy
- liver
- liver cancer
- immune checkpoint inhibitors
- tyrosine kinase inhibitors
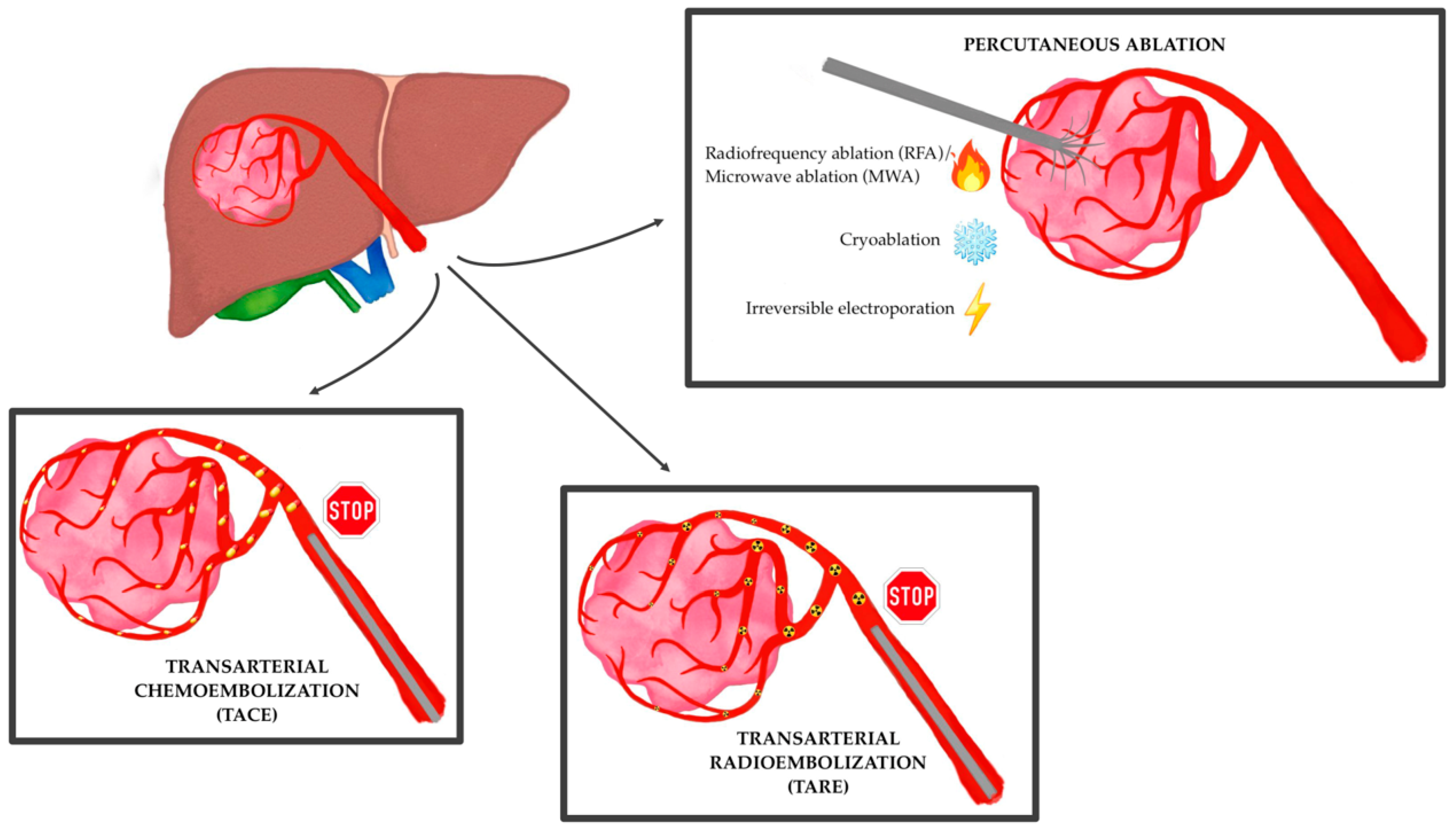
- locoregional treatment
- chemoembolization
- TACE
- tumor ablation
- radioembolization
1. The New Era in Hepatocellular Carcinoma Treatment: The Breakthrough of Immunotherapy
2. The Immunogenic Proprieties of Hepatocellular Carcinoma
HCC arises almost exclusively in the setting of chronic liver diseases, and chronic inflammation is now regarded as one of the main triggers of hepatocarcinogenesis [45,46,47][45][46][47]. Since the background of chronic inflammation promotes immune suppression, there is a tightly interwoven, exceedingly complex relationship between HCC and the anti-tumor immune response in the liver. Due to the presence of an immune-suppressed microenvironment, HCC is indeed considered an immunogenic tumor [48]. First of all, chronic inflammation plays a key role in the initiation, evolution, and progression of neoplasms by creating a microenvironment that supports the malignant transformation of hepatocytes through hepatocellular DNA damage and genetic and epigenetic aberrations [49]. When liver damage occurs, thanks to the liver’s unique considerable ability to repair itself, differentiated hepatocytes can re-enter the cell cycle and serve as their own main source of replacement [50]. However, the chronic activation of non-parenchymal cells induces altered survival and proliferation signals, resulting in cellular stress, epigenetic modifications, mitochondrial alterations, DNA damage, senescence, and chromosomal aberrations. This leads to continual cell death, compensatory regeneration and liver fibrosis, which collectively induce tumorigenesis [51]. Moreover, the increased production of pro-inflammatory cytokines occurring in the setting of chronic inflammation promotes the expression of pro-oncogenic transcription factors (such as STAT3 and NF-κB), further contributing to HCC development [52]. Secondly, chronic inflammation can boost tumor immunogenicity, creating an immunosuppressive surrounding and allowing cancer cells to escape the host immune surveillance and progress [53]. One of the main functions of the liver is to continuously remove a large and diverse spectrum of pathogen components [i.e., pathogen-associated molecular patterns (PAMP)] and endogen molecules derived from damaged or necrotized cells [i.e., damage-associated molecular patterns (DAMPs)] from the circulation, thus ensuring organ protection by maintaining immunotolerance [54]. In chronic liver diseases, however, this tightly controlled immunological network is deregulated, thus leading to the failure of efficient detection and the elimination of transformed cells and causing the breakdown of proper tolerance [53]. Once HCC has developed, an intra-tumor infiltration by lymphocytes occurs, in an attempt by the host to mediate an anti-tumor reaction [55]. Under normal circumstances, tumor antigens would be internalized by the host antigen-presenting cells (APCs) and then, after being processed, be bonded to Major Histocompatibility Complex II (MHC-II) molecules. Subsequently, if properly stimulated, dendritic cells would present these tumor antigens to T cells located in the lymphatic organs, thus promoting their activation and the stimulation of effector cells, including CD8+ T cells and Natural Killer (NK) cells. Once activated, tumor-specific effector cells would migrate from lymph nodes to the tumor location, where they would exert their cytotoxic effect on neoplastic cells. Unfortunately, these cellular responses can be dysfunctional and unable to efficiently eliminate cancer cells, thus leading to HCC progression [56]. Tumoral cells can indeed promote an elevated production of immunosuppressor cytokines (such as IL-10 and TGFβ1) that downregulate the anti-tumor response at different levels. The number of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) increases in the HCC microenvironment, which directly inhibits the tumor killing effect of NK cells and CD8+ T cells through overexpression of multiple factors [57]. In addition, MCH II is often functionally depleted in HCC, thus being unable to induce the activation of CD8+ T cells and leading to tumor immune escape [58]. Furthermore, tumoral cells inhibit the activation of APCs and promote the M2 polarization of macrophages, thus further impairing the effector functions of CD8+ T cells and NK cells [59,60][59][60]. Lastly, there is an abnormal expression and function of immune checkpoint molecules that, rather than preventing the excessive immune response from injuring normal hepatocytes as it happens in normal conditions, inhibit the host immune function and thus promote the growth of tumor cells. In particular, the most studied of them are programmed cell death protein 1 (PD-1) and its ligand (PD-L1), which leads to the T-cell exhaustion status, and cytotoxic T-lymphocyte protein 4 (CTLA-4), which inhibits the activation of T cells [61,62][61][62]. The current combined strategy of immunotherapy and locoregional treatments essentially aims to enhance the effects of immune checkpoint inhibitors (ICIs) that selectively target these immune checkpoints (PD-1/PD-L1 and CTLA-4); therefore, rather than stimulating new or different immune responses, ICIs can restore and unleash a preexisting immune reactivity to cancer which is being held in check by tumoral microenvironmental factors (Figure 2) [63,64][63][64].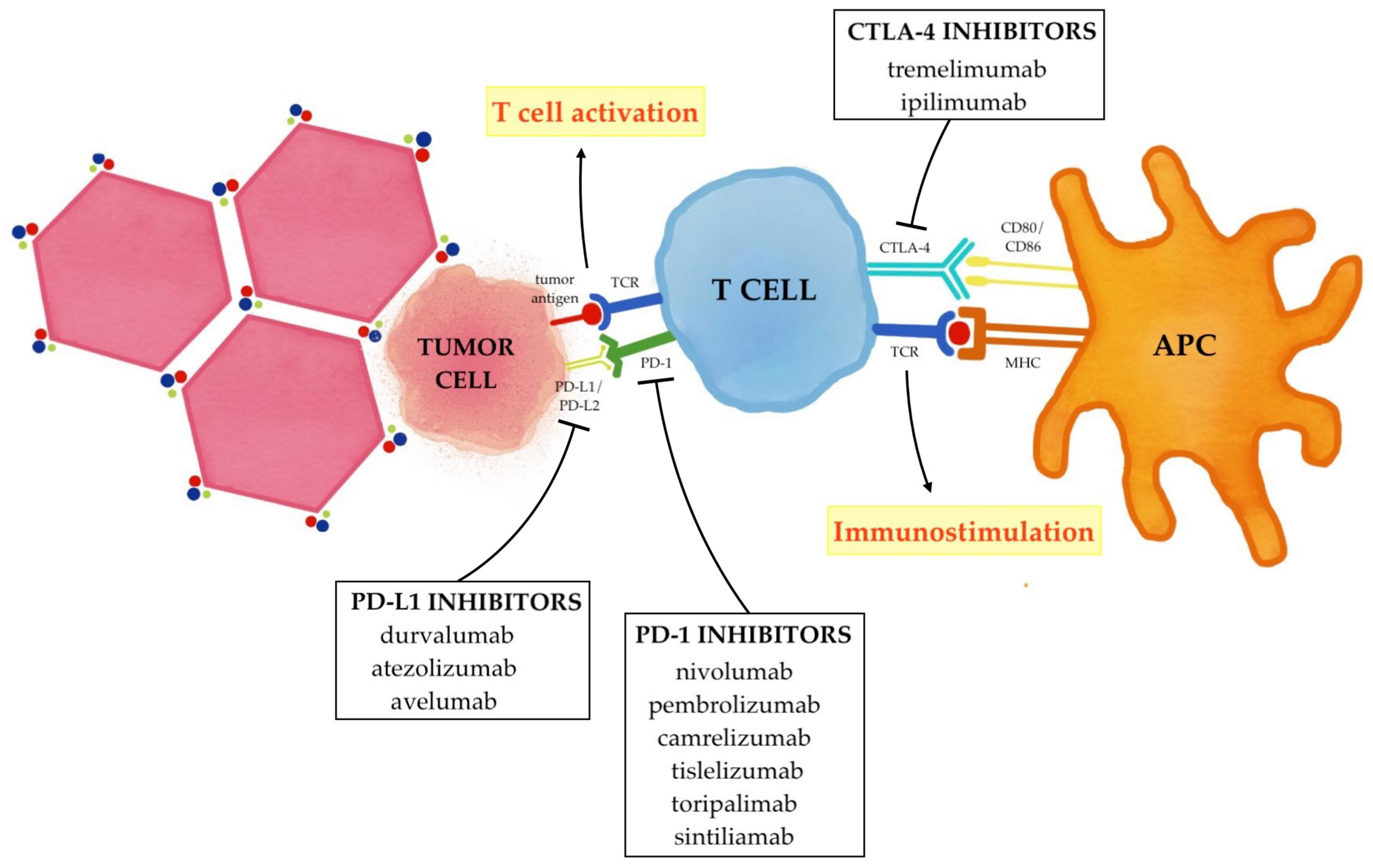
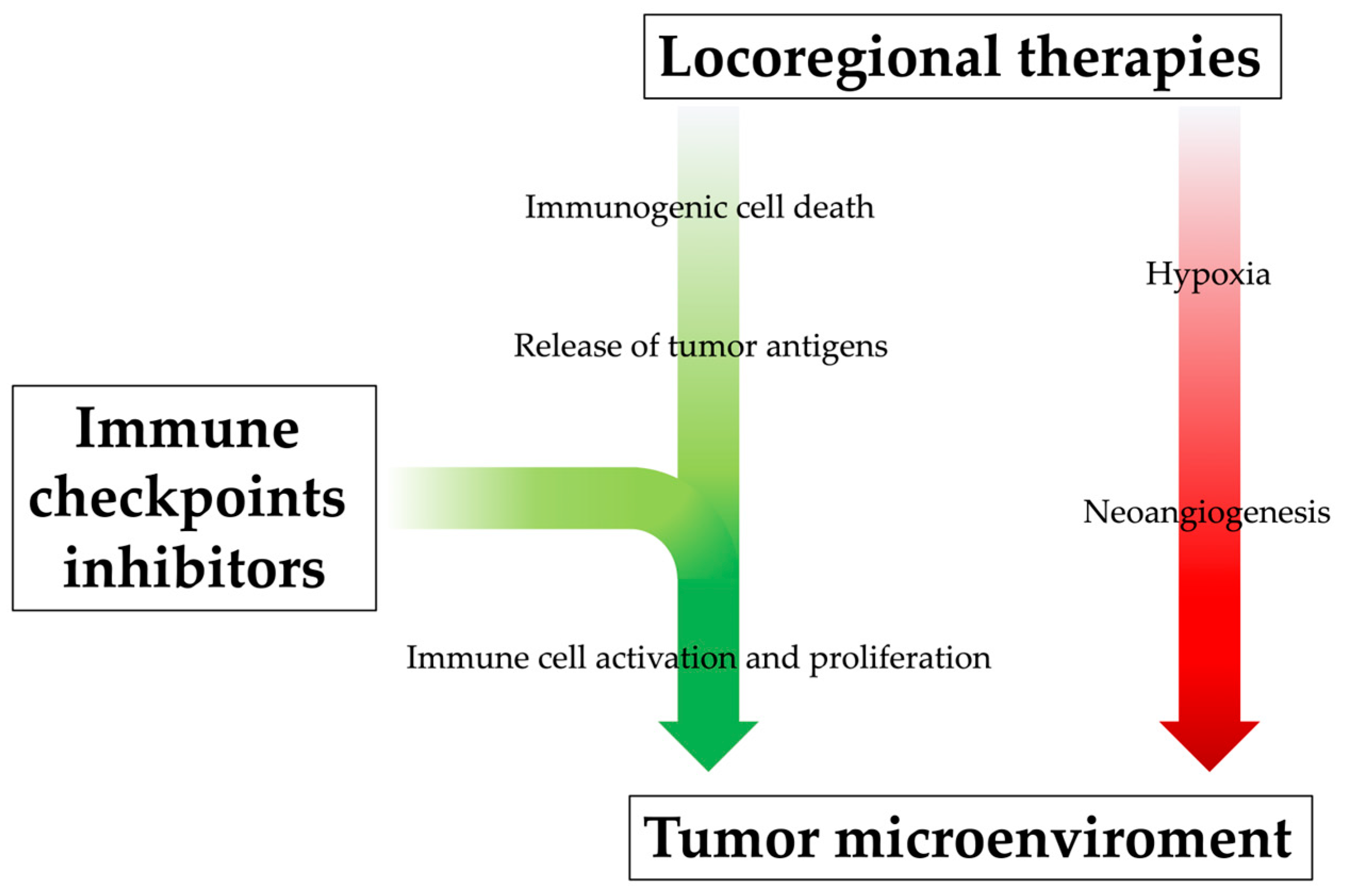
3. The Immune Modulation Effect of Locoregional Therapies
4. The Current Evidence from Clinical Trials
References
- World Health Organization. Liver Factsheet. Globocan. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 20 March 2023).
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171.
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693.
- Takayama, T.; Hasegawa, K.; Izumi, N.; Kudo, M.; Shimada, M.; Yamanaka, N.; Inomata, M.; Kaneko, S.; Nakayama, H.; Kawaguchi, Y.; et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2021, 11, 209–218.
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Ercolani, G.; Bolondi, L.; Pinna, A.D. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 2013, 59, 300–307.
- Tiong, L.; Maddern, G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011, 98, 1210–1224.
- Kong, J.; Kong, L.; Kong, J.; Ke, S.; Gao, J.; Ding, X.; Zheng, L.; Sun, H.; Sun, W. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J. Transl. Med. 2012, 10, 230.
- Park, J.; Chen, M.; Colombo, M.; Roberts, L.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166.
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal Lesions in the Cirrhotic Liver: Their Pivotal Role in Gadoxetic Acid-Enhanced MRI and Recognition by the Western Guidelines. Dig. Dis. 2014, 32, 696–704.
- Renzulli, M.; Golfieri, R. Bologna Liver Oncology Group (BLOG) Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80.
- Facciorusso, A.; Licinio, R.; Muscatiello, N.; Di Leo, A.; Barone, M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J. Hepatol. 2015, 7, 2009–2019.
- Renzulli, M.; Peta, G.; Vasuri, F.; Marasco, G.; Caretti, D.; Bartalena, L.; Spinelli, D.; Giampalma, E.; D’errico, A.; Golfieri, R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann. Hepatol. 2021, 22, 100278.
- Guarino, M.; Viganò, L.; Ponziani, F.R.; Giannini, E.G.; Lai, Q.; Morisco, F. Special Interest Group on Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig. Liver Dis. 2018, 50, 1105–1114.
- Renzulli, M.; Pecorelli, A.; Brandi, N.; Brocchi, S.; Tovoli, F.; Granito, A.; Carrafiello, G.; Ierardi, A.M.; Golfieri, R. The Feasibility of Liver Biopsy for Undefined Nodules in Patients under Surveillance for Hepatocellular Carcinoma: Is Biopsy Really a Useful Tool? J. Clin. Med. 2022, 11, 4399.
- Xue, T.-C.; Xie, X.-Y.; Zhang, L.; Yin, X.; Zhang, B.-H.; Ren, Z.-G. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: A meta-analysis. BMC Gastroenterol. 2013, 13, 60.
- Deng, J.; Liao, Z.; Gao, J. Efficacy of Transarterial Chemoembolization Combined with Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma Patients with Portal Vein Tumor Thrombus: A Systematic Review and Meta-Analysis. Curr. Oncol. 2023, 30, 1243–1254.
- Guo, L.; Wei, X.; Feng, S.; Zhai, J.; Guo, W.; Shi, J.; Lau, W.Y.; Meng, Y.; Cheng, S. Radiotherapy prior to or after transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus: A randomized controlled trial. Hepatol. Int. 2022, 16, 1368–1378.
- Geschwind, J.-F.; Kudo, M.; Marrero, J.A.; Venook, A.P.; Chen, X.-P.; Bronowicki, J.-P.; Dagher, L.; Furuse, J.; De Guevara, L.L.; Papandreou, C.; et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016, 279, 630–640.
- Wu, F.-X.; Chen, J.; Bai, T.; Zhu, S.-L.; Yang, T.-B.; Qi, L.-N.; Zou, L.; Li, Z.-H.; Ye, J.-Z.; Li, L.-Q. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer 2017, 17, 645.
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041.
- Pommergaard, H.-C.; Rostved, A.A.; Adam, R.; Thygesen, L.C.; Salizzoni, M.; Bravo, M.A.G.; Cherqui, D.; De Simone, P.; Boudjema, K.; Mazzaferro, V.; et al. Locoregional treatments before liver transplantation for hepatocellular carcinoma: A study from the European Liver Transplant Registry. Transpl. Int. 2018, 31, 531–539.
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529.
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi’s Subclassification (Kinki Criteria). Dig. Dis. 2015, 33, 751–758.
- Miyayama, S.; Matsui, O.; Taki, K.; Minami, T.; Ryu, Y.; Ito, C.; Nakamura, K.; Inoue, D.; Notsumata, K.; Toya, D.; et al. Extrahepatic Blood Supply to Hepatocellular Carcinoma: Angiographic Demonstration and Transcatheter Arterial Chemoembolization. Cardiovasc. Interv. Radiol. 2006, 29, 39–48.
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.-Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098.
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575, Erratum in Lancet Gastroenterol. Hepatol. 2017, 2, e6.
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501.
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367.
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636.
- Sangro, B.; Carpanese, L.; Cianni, R.; Golfieri, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Van Buskirk, M.; Bilbao, J.I.; et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology 2011, 54, 868–878.
- Chow, P.K.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921.
- Alonso, J.C.; Casans, I.; González, F.M.; Fuster, D.; Rodríguez, A.; Sánchez, N.; Oyagüez, I.; Burgos, R.; Williams, A.O.; Espinoza, N. Economic evaluations of radioembolization with Itrium-90 microspheres in hepatocellular carcinoma: A systematic review. BMC Gastroenterol. 2022, 22, 6.
- Radosa, C.G.; Radosa, J.C.; Grosche-Schlee, S.; Zöphel, K.; Plodeck, V.; Kühn, J.P.; Kotzerke, J.; Hoffmann, R.-T. Holmium-166 Radioembolization in Hepatocellular Carcinoma: Feasibility and Safety of a New Treatment Option in Clinical Practice. Cardiovasc. Interv. Radiol. 2019, 42, 405–412.
- Guiu, B.; Garin, E.; Allimant, C.; Edeline, J.; Salem, R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc. Interv. Radiol. 2022, 45, 1599–1607.
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352.
- Holzwanger, D.J.; Madoff, D.C. Role of interventional radiology in the management of hepatocellular carcinoma: Current status. Chin. Clin. Oncol. 2018, 7, 49.
- Aubé, C.; Bouvier, A.; Lebigot, J.; Vervueren, L.; Cartier, V.; Oberti, F. Radiological treatment of HCC: Interventional radiology at the heart of management. Diagn. Interv. Imaging 2015, 96, 625–636.
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313.
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009.
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001.
- Singh, P.; Toom, S.; Avula, A.; Kumar, V.; Rahma, O.E. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 11–17.
- Mattos, A.Z.; Debes, J.D.; Boonstra, A.; Vogel, A.; Mattos, A.A. Immune aspects of hepatocellular carcinoma: From immune markers for early detection to immunotherapy. World J. Gastrointest. Oncol. 2021, 13, 1132–1143.
- Ringelhan, M.; Pfister, D.; O’connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232.
- Cariani, E.; Missale, G. Immune landscape of hepatocellular carcinoma microenvironment: Implications for prognosis and therapeutic applications. Liver Int. 2019, 39, 1608–1621.
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570.
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641.
- Jörs, S.; Jeliazkova, P.; Ringelhan, M.; Thalhammer, J.; Dürl, S.; Ferrer, J.; Sander, M.; Heikenwalder, M.; Schmid, R.M.; Siveke, J.T.; et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J. Clin. Investig. 2015, 125, 2445–2457.
- Hernandez–Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2013, 144, 512–527.
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta 2013, 1835, 46–60.
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006.
- Crispe, I.N. The Liver as a Lymphoid Organ. Annu. Rev. Immunol. 2009, 27, 147–163.
- Qin, L.-X. Inflammatory Immune Responses in Tumor Microenvironment and Metastasis of Hepatocellular Carcinoma. Cancer Microenviron. 2012, 5, 203–209.
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased Regulatory T Cells Correlate with CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 2007, 132, 2328–2339.
- Liu, M.; Zhou, J.; Liu, X.; Feng, Y.; Yang, W.; Wu, F.; Cheung, O.K.-W.; Sun, H.; Zeng, X.; Tang, W.; et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 2020, 69, 365–379.
- Greten, T.F.; Wang, X.W.; Korangy, F. Current concepts of immune based treatments for patients with HCC: From basic science to novel treatment approaches. Gut 2015, 64, 842–848.
- Okumoto, K.; Hattori, E.; Tamura, K.; Kiso, S.; Watanabe, H.; Saito, K.; Saito, T.; Togashi, H.; Kawata, S. Possible contribution of circulating transforming growth factor-beta1 to immunity and prognosis in unresectable hepatocellular carcinoma. Liver Int. 2004, 24, 21–28.
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306.
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499.
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528.
- Johnston, M.P.; Khakoo, S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2019, 25, 2977–2989.
- Donisi, C.; Puzzoni, M.; Ziranu, P.; Lai, E.; Mariani, S.; Saba, G.; Impera, V.; Dubois, M.; Persano, M.; Migliari, M.; et al. Immune Checkpoint Inhibitors in the Treatment of HCC. Front. Oncol. 2021, 10, 601240.
- Greten, T.F.; Mauda-Havakuk, M.; Heinrich, B.; Korangy, F.; Wood, B.J. Combined locoregional-immunotherapy for liver cancer. J. Hepatol. 2019, 70, 999–1007.
- Greten, T.F.; Duffy, A.G.; Korangy, F. Hepatocellular Carcinoma from an Immunologic Perspective. Clin. Cancer Res. 2013, 19, 6678–6685.
- Pinato, D.J.; Murray, S.M.; Forner, A.; Kaneko, T.; Fessas, P.; Toniutto, P.; Mínguez, B.; Cacciato, V.; Avellini, C.; Diaz, A.; et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: Implications for immunotherapy. J. Immunother. Cancer 2021, 9, e003311.
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425, Erratum in Nat. Rev. Cancer 2015, 15, 509.
- Rubner, Y.; Wunderlich, R.; Rühle, P.-F.; Kulzer, L.; Werthmöller, N.; Frey, B.; Weiss, E.-M.; Keilholz, L.; Fietkau, R.; Gaipl, U.S. How Does Ionizing Irradiation Contribute to the Induction of Anti-Tumor Immunity? Front. Oncol. 2012, 2, 75.
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541.
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; Von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707.
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers 2020, 12, 1047.
- Birmpilis, A.I.; Paschalis, A.; Mourkakis, A.; Christodoulou, P.; Kostopoulos, I.V.; Antimissari, E.; Terzoudi, G.; Georgakilas, A.G.; Armpilia, C.; Papageorgis, P.; et al. Immunogenic Cell Death, DAMPs and Prothymosin α as a Putative Anticancer Immune Response Biomarker. Cells 2022, 11, 1415.
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500.
- Jing, X.; Zhou, Y.; Xu, X.; Ding, J.; Wang, F.; Wang, Y.; Wang, P. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J. Cancer Res. Ther. 2018, 14, 40–45.
- Zhang, H.; Hou, X.; Cai, H.; Zhuang, X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim. Invasive Ther. Allied Technol. 2017, 26, 207–211.
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457.
- Zerbini, A.; Pilli, M.; Fagnoni, F.; Pelosi, G.; Pizzi, M.G.; Schivazappa, S.; Laccabue, D.; Cavallo, C.; Schianchi, C.; Ferrari, C.; et al. Increased Immunostimulatory Activity Conferred to Antigen-presenting Cells by Exposure to Antigen Extract from Hepatocellular Carcinoma After Radiofrequency Thermal Ablation. J. Immunother. 2008, 31, 271–282.
- Ji, L.; Gu, J.; Chen, L.; Miao, D. Changes of Th1/Th2 cytokines in patients with primary hepatocellular carcinoma after ultrasound-guided ablation. Int. J. Clin. Exp. Pathol. 2017, 10, 8715–8720.
- Kohles, N.; Nagel, D.; Jüngst, D.; Stieber, P.; Holdenrieder, S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumor Biol. 2012, 33, 2401–2409.
- Liao, J.; Xiao, J.; Zhou, Y.; Liu, Z.; Wang, C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol. Med. Rep. 2015, 12, 6065–6071.
- Liao, Y.; Wang, B.; Huang, Z.-L.; Shi, M.; Yu, X.-J.; Zheng, L.; Li, S.; Li, L. Increased Circulating Th17 Cells after Transarterial Chemoembolization Correlate with Improved Survival in Stage III Hepatocellular Carcinoma: A Prospective Study. PLoS ONE 2013, 8, e60444.
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346.
- Fernandez-Ros, N.; Iñarrairaegui, M.; Paramo, J.A.; Berasain, C.; Avila, M.A.; Chopitea, A.; Varo, N.; Sarobe, P.; Bilbao, J.I.; Dominguez, I.; et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015, 35, 1590–1596.
- Seidensticker, M.; Powerski, M.; Seidensticker, R.; Damm, R.; Mohnike, K.; Garlipp, B.; Klopffleisch, M.; Amthauer, H.; Ricke, J.; Pech, M. Cytokines and 90Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc. Interv. Radiol. 2017, 40, 1185–1195.
- Zhang, J.; Li, H.; Gao, D.; Zhang, B.; Zheng, M.; Lun, M.; Wei, M.; Duan, R.; Guo, M.; Hua, J.; et al. A prognosis and impact factor analysis of DC-CIK cell therapy for patients with hepatocellular carcinoma undergoing postoperative TACE. Cancer Biol. Ther. 2018, 19, 475–483.
- Lee, H.L.; Jang, J.W.; Lee, S.W.; Yoo, S.H.; Kwon, J.H.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Han, N.I.; Yoon, S.K. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci. Rep. 2019, 9, 3260.
- Chen, M.-F.; Chen, P.-T.; Chen, W.-C.; Lu, M.-S.; Lin, P.-Y.; Lee, K.-D. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 2016, 7, 7913–7924.
- Duan, X.-H.; Li, H.; Han, X.-W.; Ren, J.-Z.; Li, F.-Y.; Ju, S.-G.; Chen, P.-F.; Kuang, D.-L. Upregulation of IL-6 is involved in moderate hyperthermia induced proliferation and invasion of hepatocellular carcinoma cells. Eur. J. Pharmacol. 2018, 833, 230–236.
- Domouchtsidou, A.; Barsegian, V.; Mueller, S.P.; Best, J.; Ertle, J.; Bedreli, S.; Horn, P.A.; Bockisch, A.; Lindemann, M. Impaired lymphocyte function in patients with hepatic malignancies after selective internal radiotherapy. Cancer Immunol. Immunother. 2018, 67, 843–853.
- Han, J.-W.; Yoon, S.-K. Immune Responses Following Locoregional Treatment for Hepatocellular Carcinoma: Possible Roles of Adjuvant Immunotherapy. Pharmaceutics 2021, 13, 1387.
- Zeng, P.; Shen, D.; Zeng, C.-H.; Chang, X.-F.; Teng, G.-J. Emerging Opportunities for Combining Locoregional Therapy with Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Curr. Oncol. Rep. 2020, 22, 76.
- Gravante, G.; Sconocchia, G.; Ong, S.L.; Dennison, A.; Lloyd, D.M. Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int. 2009, 29, 18–24.
- Nakatsura, T.; Nobuoka, D.; Motomura, Y.; Shirakawa, H.; Yoshikawa, T.; Kuronuma, T.; Takahashi, M.; Nakachi, K.; Ishii, H.; Furuse, J.; et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2012, 40, 63–70.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714.
- Wang, Y.-J.; Fletcher, R.; Yu, J.; Zhang, L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018, 5, 194–203.
- Apetoh, L.; Mignot, G.; Panaretakis, T.; Kroemer, G.; Zitvogel, L. Immunogenicity of anthracyclines: Moving towards more personalized medicine. Trends Mol. Med. 2008, 14, 141–151.
- Kloeckner, R.; Galle, P.R.; Bruix, J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 137–149.
- Cho, Y.K.; Kim, J.K.; Kim, M.Y.; Rhim, H.; Han, J.K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2008, 49, 453–459.
- Lencioni, R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010, 52, 762–773.
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626.
- Marzi, L.; Mega, A.; Gitto, S.; Pelizzaro, F.; Seeber, A.; Spizzo, G. Impact and Novel Perspective of Immune Checkpoint Inhibitors in Patients with Early and Intermediate Stage HCC. Cancers 2022, 14, 3332.
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551.
- Wang, X.; Liu, G.; Chen, S.; Bi, H.; Xia, F.; Feng, K.; Ma, K.; Ni, B. Combination therapy with PD-1 blockade and radiofrequency ablation for recurrent hepatocellular carcinoma: A propensity score matching analysis. Int. J. Hyperth. 2021, 38, 1519–1528.
- Lyu, N.; Kong, Y.; Li, X.; Mu, L.; Deng, H.; Chen, H.; He, M.; Lai, J.; Li, J.; Tang, H.; et al. Ablation Reboots the Response in Advanced Hepatocellular Carcinoma with Stable or Atypical Response During PD-1 Therapy: A Proof-of-Concept Study. Front. Oncol. 2020, 10, 580241.
- Zhang, J.-X.; Chen, P.; Liu, S.; Zu, Q.-Q.; Shi, H.-B.; Zhou, C.-G. Safety and Efficacy of Transarterial Chemoembolization and Immune Checkpoint Inhibition with Camrelizumab for Treatment of Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2022, 9, 265–272.
- You, R.; Xu, Q.; Wang, Q.; Zhang, Q.; Zhou, W.; Cao, C.; Huang, X.; Ji, H.; Lv, P.; Jiang, H.; et al. Efficacy and safety of camrelizumab plus transarterial chemoembolization in intermediate to advanced hepatocellular carcinoma patients: A prospective, multi-center, real-world study. Front. Oncol. 2022, 12, 816198.
- Ren, Y.; Liu, Z.; Makamure, J.; Kan, X.; Song, S.; Liu, Y.; Qian, K.; Zheng, C.; Liang, B. Addition of Camrelizumab to Transarterial Chemoembolization in Hepatocellular Carcinoma with Untreatable Progression. Technol. Cancer Res. Treat. 2022, 21, 15330338221131385.
- Guo, Y.; Ren, Y.; Chen, L.; Sun, T.; Zhang, W.; Sun, B.; Zhu, L.; Xiong, F.; Zheng, C. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer 2022, 22, 270.
- Ren, Y.; Guo, Y.; Chen, L.; Sun, T.; Zhang, W.; Sun, B.; Zhu, L.; Xiong, F.; Zheng, C. Efficacy of Drug-Eluting Beads Transarterial Chemoembolization Plus Camrelizumab Compared with Conventional Transarterial Chemoembolization Plus Camrelizumab for Unresectable Hepatocellular Carcinoma. Cancer Control 2022, 29, 10732748221076806.
- Tai, D.; Loke, K.; Gogna, A.; Kaya, N.A.; Tan, S.H.; Hennedige, T.; Ng, D.; Irani, F.; Lee, J.; Lim, J.Q.; et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): A single arm, single centre, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1025–1035.
- Zhan, C.; Ruohoniemi, D.; Shanbhogue, K.P.; Wei, J.; Welling, T.H.; Gu, P.; Park, J.S.; Dagher, N.N.; Taslakian, B.; Hickey, R.M. Safety of Combined Yttrium-90 Radioembolization and Immune Checkpoint Inhibitor Immunotherapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 25–34.
- De la Torre-Aláez, M.; Matilla, A.; Varela, M.; Iñarrairaegui, M.; Reig, M.; Lledó, J.L.; Arenas, J.I.; Lorente, S.; Testillano, M.; Márquez, L.; et al. Nivolumab after selective internal radiation therapy for the treatment of hepatocellular carcinoma: A phase 2, single-arm study. J. Immunother. Cancer 2023, 10, e005457, Erratum in J. Immunother. Cancer 2023, 11, e005457corr1.
- Marinelli, B.; Cedillo, M.; Pasik, S.D.; Charles, D.; Murthy, S.; Patel, R.S.; Fischman, A.; Ranade, M.; Bishay, V.; Nowakowski, S.; et al. Safety and Efficacy of Locoregional Treatment during Immunotherapy with Nivolumab for Hepatocellular Carcinoma: A Retrospective Study of 41 Interventions in 29 Patients. J. Vasc. Interv. Radiol. 2020, 31, 1729–1738.e1.
