Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marcio Vidotti and Version 2 by Beatrix Zheng.
Electrochemical sensors present a wide range of interesting applications in the areas of environmental, industrial, and chemical analysis. The use of inorganic materials is interesting due to the fact of their abundance, low cost, and good electroactivity.
- sensor
- electrocatalytic
- photoelectrocatalytic
- inorganic materials
1. Detection by Electrocatalysis
The development of efficient electrocatalysts with reduced amounts of noble metals or without noble metals is highly desirable. In this sense, organic materials, such as phthalocyanines, carbonaceous materials, and conducting polymers, have been explored as electrocatalyst materials in different electrochemical reactions. These are materials, each one with its properties, which are easy to modify and have great potential for exploration in the field of electrocatalysis.
1.1. Phthalocyanines
An organic material that arouses interest for use in electrocatalysis is phthalocyanines, which are polycyclic compounds formed by four benzene rings linked to a central porphyrin ring. To become a semiconductor, the two hydrogen atoms occupying the central ring can be replaced by a metal ion. The great stability in the redox processes of phthalocyanines attracts interest in using them in electrocatalysis processes [1][2][3][4][5][6][7][145,146,147,148,149,150,151]. Materials based on transition metal phthalocyanine have been extensively studied for the improvement of reactions involving oxygen (ORR and OER). Tajbakhsh, E., et al. used cobalt phthalocyanines in reducing carbon dioxide [2][146]. Eyele-Mezui, S. et al. used phthalocyanine tetrasulfonate intercalated in metal-layered simple hydroxides (metal: Co, Cu, and Zn) in the evaluation of the electrocatalytic activity, with a focus on fuel cells, where the materials showed potential [1][145].
Phthalocyanines combined with carbonaceous materials can improve electrocatalytic properties such as stability and selectivity. Zhang, X. et al. developed hybrid structures of cobalt phthalocyanine/carbon nanotubes for CO2 reduction, achieving an efficiency of 96% at an overpotential of 0.52 V [7][151]. Kumar, Y. et al. developed Fe-containing bimetallic–FeMn, FeNi, and FeCo–phthalocyanine complexes on multiwalled carbon nanotubes, using pyrolysis for the synthesis of mixed catalysts. The presence of the MNx catalytic center provided an increase in the oxygen reduction reaction, as well as a reduction in oxygen evolution [6][150].
1.2. Carbonaceous Materials
Carbon-based materials, such as graphene and carbon nanotubes, have a conductive surface with a high surface area. These characteristics make these materials perfect for use in electrochemical sensors and for modification by the accumulation of catalytic nanoparticles, in addition to presenting great potential as free electrocatalysts [8][9][10][11][152,153,154,155]. Li, X., et al. have successfully demonstrated the use of graphene as electrocatalysts in hydrogen evolution reactions [11][155].
Carbon nanotubes (CNTs) are nanocarbon materials with tubular structures composed of rolled-up graphene sheets. Studies using electrocatalysts based on carbon nanotubes (CNTs) demonstrate high efficiency in many typical electrocatalytic reactions. The easy structural operability and accessibility allow for the use of sustainable electrocatalysts based on these materials [12][156]. Sheng, J., et al. obtained carbon nanotubes through the pyrolysis of imidazolate zeolite structures containing Fe, and the high performance of these noble metal-free catalysts in ZABs presents great potential in real applications [13][157].
Carbon dots are another class of material that can be used in electrocatalysis. These structures can be defined as near-0D carbon-based materials with a size below 20 nm. The main characteristics of these materials are linked to fluorescence, which is their intrinsic property, in addition to having high quantum yield, low toxicity, and low cost [14][158]. Dash et al. used carbon dots to modify the glassy carbon electrodes to detect chlorpyrifos. This molecule is used in agriculture as an insecticide and is considered toxic to the environment. The authors obtained a sensor with a detection limit of 1.5 nm, with good reproducibility and good repeatability, in addition to the fact that the detection of chlorpyrifos was compared with the detections by HPLC [15][159].
These works support the great versatility of carbonaceous materials as electrocatalytic sensors and indicate the possibility of further exploring their use.
1.3. Conducting Polymers
One of the most used materials for electrocatalytic sensors is conducting polymers. Conducting polymers (CPs) are a generation of organic materials with metallic or semiconductor features, such as electrical, optical, and electronic properties, associated with the characteristics of conventional organic polymers, including ease of synthesis, corrosion resistance, flexibility, and low cost. Chemically, conductive polymers have alternating single and double carbon–carbon bonds along the polymeric chains and are considered conjugated organic polymeric entities [16][160]. By presenting this highly conjugated polymeric chain formation, these materials are extremely attractive for several applications, presenting reversible chemical, electrochemical and physical properties controlled by a doping and de-doping process [17][161].
These materials are insulators or semiconductors in the neutral or undoped form. The polymer becomes conductive only upon the removal of a p-bond electron from the conjugated polymer backbone to form a radial cation defect [18][162]. To achieve high conductivity, CPs can be doped using different methods. Due to the fact of their unique chemical structure, the doping mechanism is completely different from those occurring in semiconductor materials, where a hole is formed in the valence band. The dopants in the polymer are subjected to redox processes in which the charges are transferred with the subsequent formation of charge carriers [19][20][163,164].
The doping process occurs through chain oxidation, called p-doping, or reduction, called n-doping. In p-doping, a hole is created that causes an electron deficiency in the backbone due to the fact of electrons migrating from the HOMO of the polymeric backbone to the doping species. In n-doping, electrons are transferred to the LUMO of the polymer backbone, and electron density is generated [21][165]. In this way, these oxidation/reduction processes create charge carriers in the form of polarons (radical ions), bipolarons (dications or dianions), or solitons in the polymer.
The polarons and bipolarons can move along the polymer backbone causing localized geometric changes and are responsible for the metallic character of these materials [22][166]. The most common is p-doping, where the conjugated system stabilizes the positive charge carriers by delocalization [23][24][167,168].
Representatively, pristine undoped polypyrrole act as a semiconductor material with a bandgap of 3.16 eV. When it is p-type doping, the polymer backbone is oxidized, a π-electron is removed from the neutral PPy, and a local deformation from the benzenoid structure to a quinoid one occurs to generate a polaron. The polaron creates a localized electronic level within the band structure, and upon further oxidation, a second electron is removed resulting in the formation of doubly charged bipolarons [22][24][166,168]. In high oxidized states, overlapping bipolarons levels lead to the formation of two bipolaronic bands in the bandgap, which reduces the energy bandgap from 3.16 eV to 1.4 eV. To electrostatically balance the positive charges, a dopant anion is incorporated after the formation of a positive charge in the polymeric chain [21][165].
Compared to other organic polymers, conductive polymers have an advantage due to the fact of their adjustable chemical structure, which can be modified to change the conductivity of the polymer [16][160], allied with the low density, corrosion resistance, and control shape and morphology. Because of this, CPs have been used in several fields such as batteries [25][169], supercapacitors, sensors and biosensors [26][170], electrochromic devices [27][171], and electrocatalysts [28][172]. The most reported conducting polymers for the mentioned applications include polyaniline (PANI), polypyrrole (Ppy), and poly(3,4-ethylenedioxythiophene) (PEDOT) [29][173], as exemplified in Figure 17.
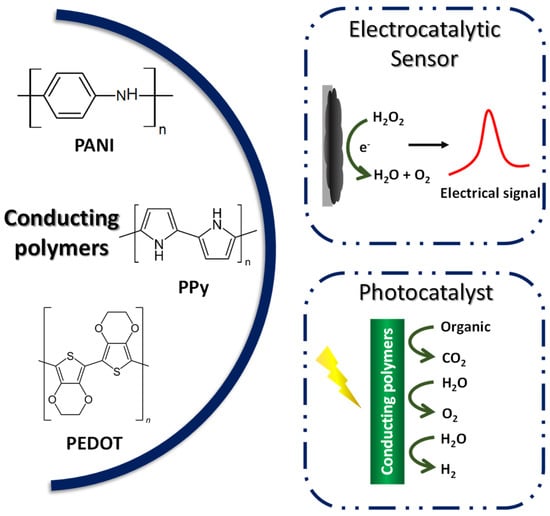
Figure 17.
Application of conductive polymers in electrocatalysis and photocatalysis.
The unique one-dimensional delocalized conjugated structures and excellent conductivity, electrochemical, and optical properties make CPs widely explored as electrocatalysis and photocatalysis materials. In addition, CP catalysts are cheap and effective and can be easily obtained on a large scale [30][174]. The inherent CP catalysts and compounds are synthesized chemically or electrochemically as powders and films. Chemical synthesis is preferable for large-scale production, while electrochemical synthesis stands out for controlling the thickness and morphology by the electrochemical parameters [21][165].
CPs have intrinsic electrocatalytic properties toward certain redox reactions. In addition, the interchain cavities that these materials present can be used for the incorporation of metal oxides and nanoparticles of catalyst metals, to generate heterogeneous electrocatalysts with enhanced catalytic activity. In addition, CPs have been used in the immobilization of redox mediators, thus increasing the electron transfer rate in catalytic cycles. Within this approach, the immobilization of biomolecules such as proteins, enzymes, or oligonucleotides stands out. Therefore, CPs demonstrate potential for use in electrocatalytic reactions, such as oxygen evolution reactions, O2 reduction, hydrogen evolution reactions, solar cells, and biosensors [23][167].
Unlike most other synthetic polymers, conductive polymers, as mentioned above, have robust conjugate structures, which are excellent candidates for electrode materials for electrocatalysis. A thin layer of a conductive polymer, deposited on the surface of the substrate electrode, can increase the kinetics of the electrode’s redox processes in some types of solution [31][32][175,176]. Three main processes are important to be considered when using conductive polymers as an electrode during the electrocatalytic conversion of solution species: (1) heterogeneous transfer of electrons between the electrode and a conductive polymeric layer and the electron transfer within the polymeric film; (2) diffusion of species from the solution so that the electrocatalytic conversion takes place; (3) chemical reaction that takes place between the solution species and the conductive polymer [32][176].
These mentioned processes can be observed in the work by Soares et al., who used PEDOT:PSS|AuNPs as an electrocatalytic sensor in catechol detection. The modified electrode showed a high electrochemical response and strong interactions with the analyte, which allowed for the development of different forms of transduction based on electrochemical reactions and changes in Rct values. In the work, the strong catechol adsorption on the substrate was attributed to hydrogen bonding in the oxyethylene ring of PEDOT, confirmed by Raman spectroscopy [33][177].
The dopant used in polymer synthesis can directly influence the conductivity of the material. For example, when PEDOT polymer is doped with an anionic system, the high density of the positive charge carriers within the PEDOT phase, necessary for electronic conductivity, is enabled by the charge compensation of the anions. The polymer maintains its electroneutrality by the insertion of anions or by the expulsion of cations. When using large dopants that turn out to be immobile, such as polystyrenesulfonate (PSS), the ionic charge compensation is mainly provided by mobile cations of the electrolyte, which makes cation transport dominant in PEDOT:PSS during oxidation–reduction exchange. When the anions used are small and exchangeable (for example, tosylate (Tos)), it results in an electrode that favors the transport of anions to balance the charge compensation [31][34][35][36][175,178,179,180].
Another advantage of CPs over conventional metal electrocatalysts is that they can be used to improve sensor selectivity. This advantage was explored in the work of Tang et al. [37][181], where the efficient junction of the characteristics of Ppy, such as hydrophobicity and π bonds, combined with good electrical conductivity of graphene oxide, was used to prepare a selective sensor for NH3. For the sensor construction, a thin film of Ppy was electrodeposited in reduced graphene oxide (rGO). The sensor demonstrated a fast, reversible, and linear response in the range of 1 to 4 ppm, with excellent selectivity of 6.1% ppm for ammonia detection. The performance obtained by the Ppy/rGO sensor is due to the synergistic effect between the ultrathin layer of Ppy and rGO. The rGO sheets, in addition to acting as a support for Ppy, allow an efficient path for electron transfer, accelerating the response and sensor recovery, while the hydrophobic nature of Ppy protects the sensor from humidity. Still, the excellent selectivity of the sensor was attributed to the adsorption of NH3 molecules in the ultrathin layer of Ppy and electron transfer between the NH3 and Ppy layers.
Despite all the catalytic properties of interest, CPs still have some disadvantages. For example, the low ion transfer rate during the redox reaction eventually leads to area capacitance saturation as the polymer film thickness increases [38][182]. To overcome this limitation, the introduction of other materials, such as metallic oxides is interesting. Shyamala, S. et al. used this strategy of doping polyaniline nanocomposites with zirconia in the oxidation of formic acid (FAO) for an ethanol oxidation reaction (EOR) [38][182]. Uwaya, G. E. et al. used polyaniline/nickel oxide in the detection of epinephrine (EP), where the obtained nanocomposite exhibited a better electrocatalytic response with a greater current response and a lower resistance to charge transfer [39][183].
Another approach to improve the catalytic activity and conductivity are the introduction of metal nanoparticles (e.g., Au and Pt) or carbon nanomaterials in the polymer matrix [16][160], resulting in hybrid catalysts. This type of catalyst will be further discussed in the next section.
2. Detection by Photoelectrocatalysis
Among the organic semiconductors, carbon nitrides stand out, having achieved a lot of interest in recent years, as well as their application as sensors [40][41][42][12,184,185]. g-C3N4 consists of a polymeric material that contains nitrogen (N) and carbon (C) atoms with sp2 hybridization and forms a highly delocalized conjugate system connected via s-triazine or s-heptazine motifs [43][186].
Li et al. [43][186] investigated a photoelectrochemical detection platform for the highly sensitive and selective detection of gallic acid, based on the g-C3N4@CNT heterojunction. This research is interesting, as gallic acid is found in a wide range of natural plants and is relevant to the health of humans. The results display that abundant amino groups of g-C3N4 provide excellent selectivity for the sensor. In addition, the sensor was investigated for the analysis of GA in black tea samples, providing a novel and quick method for the detection of GA in food samples.
Li et al. [44][187] show the detection of metronidazole (MNZ) using a simple, easy, and sensitive photoelectrochemical bioassay (PEC) protocol. In this research, samples of common oral drugs were used under visible light irradiation, where new g-C3N4 nanoarchitectures similar to hierarchical corals (cg-C3N4) were explored for the first time as a PEC detection platform.
