1. The New Era in Hepatocellular Carcinoma Treatment: The Breakthrough of Immunotherapy
Hepatocellular carcinoma (HCC) is the sixth most commonly occurring cancer worldwide, and due to its constantly increasing incidence, it has become the third leading cause of cancer-related death among general populations. Moreover, it represents the most common cause of death in patients with cirrhosis
[1][2][1,2]. Multiple classification schemes are available to stratify HCC patients in an effort to determine which therapies they can undergo to increase their overall survival. The Barcelona Clinic Liver Cancer Staging (BCLC) system is one of the most widely used, and takes into account hepatic function, the extent of tumor involvement, and performance status
[3].
The definitive therapies for HCC remain surgical resection and liver transplantation that can be performed only in patients at very early (0) and early (A) stages. However, given the similar survival benefit paired with the less invasiveness and lower costs compared to surgical resection, percutaneous ablative therapies such as radiofrequency ablation (RFA) and microwave ablation (MWA) are now considered the first treatment approach in both very early and early stages, especially in patients with small HCC (≤3 cm)
[4][5][4,5]. Despite inducing an effective local antitumor effect, the responses to ablation techniques are relatively weak and might not completely control the tumor, as testified to by the high local recurrence rates. In particular, the size, number and location of tumors can be responsible for incomplete treatment response
[6]; in addition, by promoting angiogenesis of residual cancer cells through both transcriptional and epigenetic regulations, insufficient ablation could lead to the recurrence of HCC with a more aggressive phenotype
[7]. Therefore, novel techniques to improve ablation efficacy are currently being investigated.
Despite the improvement in screening and surveillance programs, most patients with HCC (about 65–70%) are still diagnosed in the intermediate (B) or advanced (C) tumoral stages, and are thus ineligible for radical therapies
[8][9][10][8,9,10]; therefore, patients with intermediate and/or advanced HCCs are considered for transarterial therapies or systemic therapies
[11][12][11,12] which, albeit effective, are deemed non-curative or “palliative” and still yield a lower 5-year survival rate
[13][14][13,14]. According to BCLC tumor staging and management
[3], transarterial chemoembolization (TACE) is recommended as first-line therapy for unresectable intermediate-stage HCC (stage B). Therefore, it is not surprising that this treatment was the most widely used first line treatment for the treatment of HCC across the world. More interestingly, instead, TACE emerged as the most frequently used first-line treatment for early and advanced stages, thus making it the most frequent treatment for HCC overall
[8]. In fact, TACE is potentially suitable and safe for selected patients in the advanced stage with tumor vein thrombosis
[15][16][17][15,16,17], or in combination with systemic therapies, without safety concerns
[18][19][18,19]. Additionally, TACE can be safely and effectively performed in patients at very early and early stages that are partial responders to surgery or ablation, or that are unfit for these curative therapies due to contraindications
[20], or prior to liver transplantation to downstage the tumor burden
[21]. Despite evidence of beneficial short-term outcomes with locoregional treatments, recurrence and distant metastasis continue to have a significant effect on the overall survival of patients with HCC, especially in intermediate and advanced stages. This may be partly explained by the hypoxic environment created by the TACE procedure, which can induce neoangiogenesis by stimulating vascular endothelial growth factor (VEGF) and other angiogenic pathways, promoting revascularisation and growth of residual viable tumors or even new lesions
[22]. Moreover, when it comes to transarterial therapies, one important consideration is that the blockade of hepatic arteries, especially if repeated several times, can compromise liver function and lead to collateral vessel formation, thus limiting the ability to repeat embolization by conventional hepatic vasculature
[23][24][23,24]. In an effort to address this problem, many studies have been conducted combining TACE with systemic anti-angiogenic agents, most commonly sorafenib, with the aim to counteract this paradoxical effect and thus extend the clinical benefit derived from TACE. However, although most of these studies report the safety of the combination
[18], a large number of clinical trials have failed to demonstrate any significant improvement in clinically relevant outcomes for patients with intermediate-stage HCC
[25][26][25,26]. Even the more recent TACTICS trial, despite being the first study to demonstrate a longer progression-free survival (PFS) in patients receiving sorafenib plus TACE than in those receiving TACE plus placebo
[27], it did not significantly extend overall survival (OS) in its final post-hoc analysis
[28]. Therefore, better strategies to improve the outcomes for HCC patients treated with TACE are being developed.
Along with TACE, the role of other radiological locoregional therapies has expanded in recent years. For example, transarterial radioembolization (TARE) with yttrium-90 has been suggested as a safe and effective alternative treatment option for HCC patients with a liver-dominant disease who cannot tolerate systemic therapies
[29][30][31][29,30,31], even with a significant cost advantage
[32]. Moreover, the recent availability of new microspheres with a different radioisotope (such as 166-holmium) and the new technological developments will probably contribute to further reinforce the role of this option in HCC treatment and expand its clinical indication even in early and intermediate stages
[33][34][33,34]. Nonetheless, due to the current lack of evidence demonstrating its superiority and non-inferiority to sorafenib, TARE is now recommended only in single HCCs ≤ 8 cm
[3][35][3,35], and its role behind this indication remains uncertain.
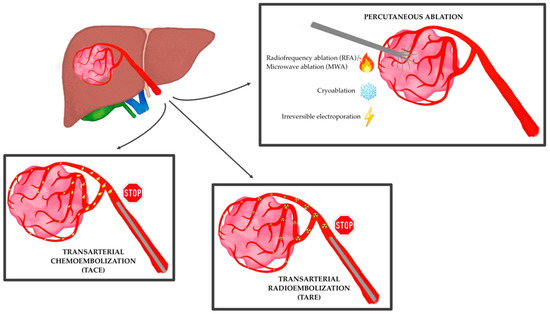
Therefore, despite current limitations, the role of interventional radiology in the treatment of HCC is continuing to grow at each stage of the disease, especially at centers of excellence with multidisciplinary tumor boards, whether it is performed with curative, downstaging, bridging, debulking or palliative intent (
Figure 1)
[36][37][36,37]. Moreover, its expansion is expected to further progress as technical and clinical innovation continue to outpace large randomized controlled trials, with 50–60% of HCC patients that are expected to receive these treatments in their lifespan, globally
[38].
Figure 1.
The main locoregional techniques for the treatment of hepatocellular carcinoma.
In recent years, immunotherapy has led to a major shift in the treatment of HCC and prompted clinical trials, with therapeutic agents being used to selectively target immune cells rather than cancer cells
[39]. In particular, the combination of atezolizumab and bevacizumab is now regarded as the standard first-line treatment for patients with advanced HCC due to the significant and clinically meaningful improvements in terms of OS, PFS, objective response rate (ORR) and complete response rate (CRR) compared with sorafenib monotherapy
[3][40][41][3,40,41]. More recently, the combination of tremelimumab and durvalumab has been reported to be superior to sorafenib in patients with advanced or unresectable HCC, adding another first-line treatment option
[42]. The impressive benefit provided by immunotherapy in patients with advanced HCC has led to the question if there is a rationale to support the combination of these new drugs with locoregional therapies in an adjuvant or neoadjuvant setting even in the early and/or intermediate stages
[43]. In fact, it has now been demonstrated that locoregional treatments can positively alter the immune microenvironment of HCC and, theoretically, have a synergistic effect, further enhancing antitumor immune responses and thus improving patient survival
[44].
2. The Immunogenic Proprieties of Hepatocellular Carcinoma
HCC arises almost exclusively in the setting of chronic liver diseases, and chronic inflammation is now regarded as one of the main triggers of hepatocarcinogenesis
[45][46][47][45,46,47]. Since the background of chronic inflammation promotes immune suppression, there is a tightly interwoven, exceedingly complex relationship between HCC and the anti-tumor immune response in the liver. Due to the presence of an immune-suppressed microenvironment, HCC is indeed considered an immunogenic tumor
[48].
First of all, chronic inflammation plays a key role in the initiation, evolution, and progression of neoplasms by creating a microenvironment that supports the malignant transformation of hepatocytes through hepatocellular DNA damage and genetic and epigenetic aberrations
[49]. When liver damage occurs, thanks to the liver’s unique considerable ability to repair itself, differentiated hepatocytes can re-enter the cell cycle and serve as their own main source of replacement
[50]. However, the chronic activation of non-parenchymal cells induces altered survival and proliferation signals, resulting in cellular stress, epigenetic modifications, mitochondrial alterations, DNA damage, senescence, and chromosomal aberrations. This leads to continual cell death, compensatory regeneration and liver fibrosis, which collectively induce tumorigenesis
[51]. Moreover, the increased production of pro-inflammatory cytokines occurring in the setting of chronic inflammation promotes the expression of pro-oncogenic transcription factors (such as STAT3 and NF-κB), further contributing to HCC development
[52].
Secondly, chronic inflammation can boost tumor immunogenicity, creating an immunosuppressive surrounding and allowing cancer cells to escape the host immune surveillance and progress
[53]. One of the main functions of the liver is to continuously remove a large and diverse spectrum of pathogen components [i.e., pathogen-associated molecular patterns (PAMP)] and endogen molecules derived from damaged or necrotized cells [i.e., damage-associated molecular patterns (DAMPs)] from the circulation, thus ensuring organ protection by maintaining immunotolerance
[54]. In chronic liver diseases, however, this tightly controlled immunological network is deregulated, thus leading to the failure of efficient detection and the elimination of transformed cells and causing the breakdown of proper tolerance
[53]. Once HCC has developed, an intra-tumor infiltration by lymphocytes occurs, in an attempt by the host to mediate an anti-tumor reaction
[55]. Under normal circumstances, tumor antigens would be internalized by the host antigen-presenting cells (APCs) and then, after being processed, be bonded to Major Histocompatibility Complex II (MHC-II) molecules. Subsequently, if properly stimulated, dendritic cells would present these tumor antigens to T cells located in the lymphatic organs, thus promoting their activation and the stimulation of effector cells, including CD8+ T cells and Natural Killer (NK) cells. Once activated, tumor-specific effector cells would migrate from lymph nodes to the tumor location, where they would exert their cytotoxic effect on neoplastic cells. Unfortunately, these cellular responses can be dysfunctional and unable to efficiently eliminate cancer cells, thus leading to HCC progression
[56].
Tumoral cells can indeed promote an elevated production of immunosuppressor cytokines (such as IL-10 and TGFβ1) that downregulate the anti-tumor response at different levels. The number of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) increases in the HCC microenvironment, which directly inhibits the tumor killing effect of NK cells and CD8+ T cells through overexpression of multiple factors
[57]. In addition, MCH II is often functionally depleted in HCC, thus being unable to induce the activation of CD8+ T cells and leading to tumor immune escape
[58]. Furthermore, tumoral cells inhibit the activation of APCs and promote the M2 polarization of macrophages, thus further impairing the effector functions of CD8+ T cells and NK cells
[59][60][59,60]. Lastly, there is an abnormal expression and function of immune checkpoint molecules that, rather than preventing the excessive immune response from injuring normal hepatocytes as it happens in normal conditions, inhibit the host immune function and thus promote the growth of tumor cells. In particular, the most studied of them are programmed cell death protein 1 (PD-1) and its ligand (PD-L1), which leads to the T-cell exhaustion status, and cytotoxic T-lymphocyte protein 4 (CTLA-4), which inhibits the activation of T cells
[61][62][61,62].
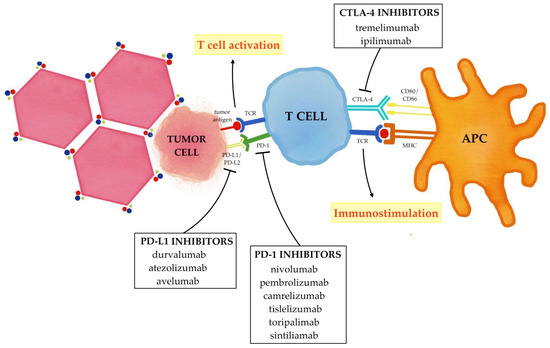
The current combined strategy of immunotherapy and locoregional treatments essentially aims to enhance the effects of immune checkpoint inhibitors (ICIs) that selectively target these immune checkpoints (PD-1/PD-L1 and CTLA-4); therefore, rather than stimulating new or different immune responses, ICIs can restore and unleash a preexisting immune reactivity to cancer which is being held in check by tumoral microenvironmental factors (
Figure 2)
[63][64][63,64].
Figure 2. Immune checkpoint inhibitors in hepatocellular carcinoma. PD-1 binding its ligand PD-L1/PD-L2 prevents TCR signaling, blocks T cell proliferation, and induces the exhaustion of T cells. CTLA-4 binds CD80/CD86 and blocks the activation of the T cells. The inhibition of these immune checkpoints with PD-1/PD-L1 inhibitors and CTLA-4 inhibitors promote T cell activation and up-regulate the immune system, thus reactivating the anticancer immune response.
3. The Immune Modulation Effect of Locoregional Therapies
In several animal and human studies, locoregional treatments have been shown to induce immune responses in HCC patients, positively altering their tumor microenvironment
[65][66][84,85]. The release of tumor antigens due to cell death and subsequent recruitment and activation of APCs and effector immune cells are the main processes responsible for the changes in anti-tumor immune responses after locoregional treatments
[65][84].
Immunogenic cell death involves the translocation of calreticulin on the cell surface, the secretion of ATP, and the release of the non-histone chromatin protein high-mobility group box 1 (HMGB1) and other immunostimulatory molecules that collectively facilitate the recruitment and activation of APCs into the tumor microenvironment, the engulfment of tumor antigens from dying tumor cells and, finally, the optimal antigen presentation to T cells
[66][67][68][69][70][71][85,86,87,88,89,90]. Locoregional treatments can induce both apoptosis and necrosis of tumor cells. Necrosis is a form of cell death characterized by loss of plasma membrane integrity, culminating in the escape of cell contents into the extracellular space, including tumor specific antigens, thus is known to be immunogenic; conversely, apoptosis is a programmed cell death in which the plasma membrane is not disrupted and cellular contents are packaged and then released into apoptotic bodies, thus it is regarded as immunologically “silent”
[72][73][91,92]; nevertheless, previous reports have also implicated that certain types of apoptosis could be immunogenic and therefore favor the immune response against the tumor
[74][75][93,94].
A plethora of cytokines, chemokines, and inflammatory/cell stress molecular markers have been described following the execution of the majority of locoregional treatments for HCC, supporting the immune modulation effect of these techniques. The effect of MWA as a single therapy was one of the first to be investigated, demonstrating the activation of Tregs, CD4+ and CD8+ T cell and NK cells, as well as the release of IL-12
[76][77][95,96]. The evidence that ablative therapy can cause tumor-specific immune responses was observed also in patients who underwent RFA, which can increase the number of tumor-associated antigen-derived peptides in peripheral blood
[78][97], induce APCs activation and proliferation
[79][98] and stimulate the secretion of Th1 cytokines (such as IL-2, TNF-α and IFN-γ) that promote CD8+ T activity
[80][99]. Similarly, also TACE was reported to promote immunogenic cell death, as testified to by the increased serum levels of immunogenic cell death biomarkers following the procedure
[81][100]; moreover, TACE can also promote Th17 and CD8+ activation and reduce the number of Tregs
[82][83][101,102]. More recently, infiltration of CD8+ T cells and NK cells and an increase in cytokines levels (especially IL-1, IL-6 and IL-8) was found after TARE with yttrium-90
[84][85][86][103,104,105].
Locoregional therapy can promote systemic immune response by releasing neoantigens into blood circulation, but their effect alone might be too modest to prevent tumor recurrence and metastasis, even after successful treatments. Moreover, especially when incomplete, locoregional treatments can also induce immunosuppressive factors (such as IL-6, VEGF, HIF-1α, TGF-β, PD-1 and PD-L1), stimulate the accumulation of Tregs in the tumor and cause lymphopenia, leading to tumor progression in the end
[87][88][89][90][91][106,107,108,109,110]. Incomplete T cell restoration despite antigen clearance and immune-tolerant liver environment might also affect the attenuation of immune surveillance. Additionally, their immunological effects appear limited in time. Indeed, as demonstrated by a previous study, the memory phenotype and lifetime of tumor-specific T cells were not sufficient to prevent HCC recurrence completely after RFA
[78][97]. For all these reasons, the efficacy of locoregional treatments could be enhanced by their combination with immunotherapeutic drugs, which would guarantee the achievement of an immunologically more favorable tumor microenvironment
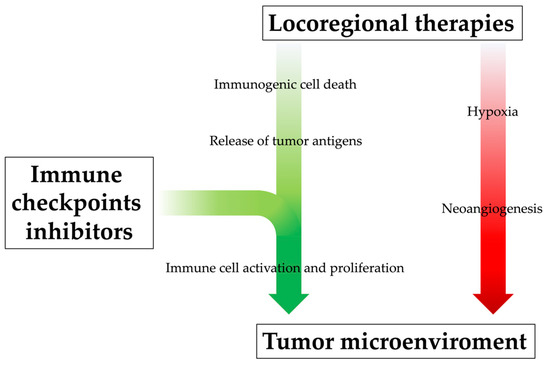
[92][93][111,112]; at the same time, through a mutually beneficial and synergistic mechanism, the positive alteration of the tumor microenvironment derived from locoregional treatments may enhance ICI therapy efficacy (
Figure 3)
[38].
Figure 3. The rationale behind combining locoregional therapies and immunotherapy. Locoregional therapies, especially when incomplete, can increase the level of pro-angiogenetic cytokines and thus promote neoangiogenesis of residual cancer cells and metastasis development; at the same time, however, they promote systemic immune response by releasing neoantigens into blood circulation, although this immunogenic effect might be too modest. The immunological efficacy of locoregional treatments could be enhanced by their combination with immunotherapeutic drugs, which would promote immune cell activation and proliferation, positively influencing the tumor microenvironment.
To date, there is no direct comparison between the different ablation or intra-arterial techniques, therefore it is not known whether one technique is superior to the others in inducing tumor-specific immune response
[94][113]. In a previous study, it was demonstrated that serum levels of Glypican-3, a carcinoembryonic antigen inducing tumor-specific activation of cytotoxic T cells, were increased in 55% of patients with HCC after RFA and in 44% of patients after TACE, although these results were non-significant
[95][114]. Interestingly, more recent evidence seems to suggest that TACE may have a greater immunogenic role than other locoregional treatments, possibly due to the potential immunogenic cell death induced by doxorubicin
[96][115]. Doxorubicin is the most used chemotherapeutic agent for TACE and, despite the absence of a proven superiority compared to other drugs (such as cisplatin, epirubicin and mitomycin), is the only one to have demonstrated to possess immunogenic properties and thus trigger a significant tumor-specific immunological response
[97][116]. In particular, anthracyclines such as doxorubicin seem to cause the post-transcriptional translocation of calreticulin from the endoplasmic reticulum, where it is involved in the maintenance of Ca
2+ homeostasis, to the plasma membrane of tumor cells; surface-exposed calreticulin then acts as an “eat me” signal for phagocytosis by neighboring APCs, which is required for subsequent antigen cross-presentation to cytotoxic T cells
[98][117]. Because chemotherapy is an integral part of TACE, these studies indicate that not only the immunogenic effects of embolization must be considered, but also the immune effects of the chemotherapy of choice. Therefore, if TACE is combined with immunotherapy, doxorubicin likely would lead to better outcomes compared to other chemotherapeutic agents.
4. The Current Evidence from Clinical Trials
The high risk of local and distant recurrence after locoregional treatments indicates the need for efficient adjuvant strategies to improve cure rates, even at very early and early stages. Features, such as large tumors, multinodularity, and vascular invasion (macroscopic or microscopic), are significantly related to higher recurrence rates in both ablative and intra-arterial therapies
[99][100][101][118,119,120]. With this perspective, the addition of immunotherapy after locoregional treatments could amplify the effect of these treatments against micro-metastatic residual disease, especially in patients with a high risk of recurrence or those who would present clinical or hepatic deterioration after treatment. Similarly, there is a rationale to integrate immunotherapy in the neoadjuvant setting as well, especially in intermediate and advanced stages. The pre-treatment administration of ICIs can indeed leverage the higher levels of tumor antigens and thus promote the expansion of tumor-specific T cells, increasing the chance of cure following locoregional treatments
[102][103][121,122].
One of the first trials that investigated the role of ICIs in combination with locoregional treatments in HCC patients evaluated the safety and efficacy of tremelimumab plus subtotal conventional TACE, RFA or cryoablation in patients who were non-responders to sorafenib. In particular, the protocol was shown to be safe and feasible, with no clear trends in adverse events or dose-limited toxicity; moreover, this therapeutic combination resulted in objective tumor responses even outside of the ablated or embolized zone, indicating that the systemic effects brought by locoregional therapies indeed exist
[104][123]. The combination of tremelimumab plus ablation (RFA or cryoablation) or drug-eluting beads TACE (DEB-TACE) was also assessed in another study with HCC patients progressed on sorafenib therapy, proving the safety and efficacy of the protocol; in particular, the primary lesion kept shrinking and almost disappeared at 6 months and the untreated other intrahepatic lesions reduced in size gradually
[65][84]. The enhanced efficacy of anti-PD-1 and ablative combined therapy was later confirmed in another retrospective study, where patients who underwent RFA plus camrelizumab or sintilimab demonstrated a longer OS and a higher recurrence-free survival (RFS) compared to those treated with RFA alone (32.5% vs. 10.0% and 51 weeks vs. 47.6 weeks, respectively)
[105][124]. Similarly, a proof-of-concept clinical trial enrolling 50 patients with advanced HCC after sorafenib failure reported that additional RFA or MWA to anti-PD-1 therapy (nivolumab or pembrolizumab) increased the response rate from 10% to 24%. This latter study, moreover, documented that repeated ablations were also proved feasible and safe, reporting only common ablation-related complications that were easily managed as per the standard of care
[106][125].
Three different studies
[107][108][109][126,127,128] indicated that anti-PD-1 therapy (camrelizumab) plus TACE regimen is effective and safe, with effective tumor control, improved survival and manageable ICI-related adverse effects, leading to better outcomes than treatment with anti-PD-1 inhibitors alone; moreover, a longer interval between camrelizumab administration and TACE was related to the unsatisfying OS, whereas the timing of administration (before or after TACE) did not significantly influence the results. However, another study reported similar efficacy of TACE combined with camrelizumab compared to TACE alone, although the protocol was safe and tolerable
[110][129]. Among the most common adverse events, itching was the most common, and is often associated with dermatitis and increased liver transaminases; whereas the appearance of colitis, thyroiditis and pneumonia is rarer. An interesting study compared the efficacy and safety of conventional TACE + camrelizumab with DEB-TACE + camrelizumab with the aim of determining which technique was superior. Despite both protocols being safe and well-tolerated, DEB-TACE produced better tumor response and PFS (70.4% vs. 40.7% and 10 vs. 3 months, respectively); however, these results could have been influenced by the inclusion of patients with large and multiple HCCs, who are theoretically more susceptible to this type of intra-arterial procedure; thus, further studies are needed
[111][130].
Similar to TACE, even TARE in combination with nivolumab was demonstrated as a safe and effective treatment for HCC patients, showing a higher objective response rate (ORR) compared to both TARE alone and anti-PD-1 agents alone (30.6% vs. 20% vs. 15–23%, respectively)
[112][131]; of note, the ORR in patients without extrahepatic spread was 43.5%, suggesting that TARE followed by nivolumab should be further evaluated in patients with BCLC B or BCLC C with no extrahepatic spread. One small retrospective trial examined patients with advanced HCC but preserved liver function who had received TARE and nivolumab with or without ipilimumab, documenting the safety of this association; moreover, there were no differences in toxicities between patients who received both therapies within 30 days of each other and those who received both therapies within 30–90 days
[113][132]. The safety and efficacy of TARE plus anti-PD-1 therapy were also confirmed in other studies
[114][115][133,134].
Despite this encouraging evidence, larger and comparative studies are needed to confirm the efficacy of immunotherapy combined with locoregional treatments in HCC patients. Currently, several other trials are exploring the role of ICIs in combination with locoregional treatments in HCC patients, with or without other drugs (such as tyrosine-kinase inhibitors), but participants are still being recruited or are receiving intervention, or data have yet to be analyzed. The role of numerous immunotherapeutic drugs is being tested in the adjuvant setting of patients who underwent ablative therapies, including nivolumab (the CheckMate 9DX trial, NCT03383458), atezolizumab plus bevacizumab (the IMbrave050 trial, NCT04102098) and pembrolizumab (the KEYNOTE-937 trial, NCT03867084); similarly, the use of nivolumab in both adjuvant and neoadjuvant settings after electroporation is currently being investigated (the NIVOLEP trial, NCT03630640). The number of studies that are evaluating the combination of immunotherapy with TACE and TARE in intermediate and advanced patients is even larger. The results of the LEAP-012 trial exploring TACE plus pembrolizumab plus lenvatinib in advanced HCC patients are eagerly awaited (NCT04246177), as are those of ongoing trials evaluating TACE plus atezolizumab plus bevacizumab (NCT04712643), TACE plus durvalumab plus bevacizumab (the EMERALD-1 trial, NCT03778957), TACE plus durvalumab plus bevacizumab plus tremelimumab (the EMERALD-3 trial, NCT05301842), TACE plus nivolumab (the TACE-3 trial, NCT04268888) and TACE plus nivolumab plus ipilimumab (the CheckMate 74W trial, NCT04340193). Similarly, the results that will emerge from trials combining TARE with yttrium-90 plus nivolumab (NCT03033446), pembrolizumab (NCT03099564) or durvalumab plus tremelimumab (the MEDI4736 trial, NCT04522544) are highly anticipated.