Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 1 by Rui Alvites.
The Olfactory Bulb is a component of the Olfactory System, in which it plays an essential role as an interface between the peripheral components and the cerebral cortex responsible for olfactory interpretation and discrimination. It is in this element that the first selective integration of olfactory stimuli occurs through a complex cell interaction that forwards the received olfactory information to higher cortical centers.
- Olfactory Bulb
- olfactory system
- olfaction
1. Introduction
The olfactory system, as one of the five traditional sensory senses, is of fundamental importance in animal behavior and in its interaction with the surrounding environment. Its functions extend to roles as diverse as feeding and foraging, inter and intraspecific communication, exploration, and environmental recognition, predation or avoidance of predators, mating and reproduction, and territorial marking [1]. The capture, recognition, and processing of odorant molecules results from an interaction between different components of the olfactory system. The Olfactory Bulb (OB) is part of the main olfactory system and works as an intermediate component between the olfactory epithelium in the nasal cavity and the connections established with distinct parts of the brain. This component is a multilayered structure contained in the central nervous system that receives the information contained in the odoriferous stimuli captured by the olfactory receptors peripherally. These signals are transmitted in a hierarchical and highly organized way through complex interactions between primary and secondary neurons within the so-called olfactory glomeruli, and the properly filtered information is further transmitted to higher cortical levels [2]. The OB is one of the first structures to evolutionarily appear in the vertebrate brain [3], and its importance justifies the fact that, although neurogenesis in the adult brain registers mainly in the hippocampus and subventricular zone [4], the OB receives neuroblasts through the rostral migratory stream, allowing it to refine the animal’s sensitivity to smells [5].
2. Anatomy and Structural Organization of the Olfactory Bulb
The OB is a bulbar structure located in the rostral extremity of each cerebral hemisphere, ventrocranially to the frontal lobe [25][6] and near the orbital portion of the frontal bone, arranged on each of the ethmoidal fossae of the cribriform plate through which it receives the constituent filaments of the olfactory nerve. The two OBs are separated from each other by the crista galli of the ethmoid bone. In the dog it has a volume of around 1.8 mm3 in normosmic individuals, representing around 0.31% of the total brain volume; this percentage drops to 0.01% in the human brain. Integrated in the respective cerebral hemisphere, the OB represents around 43% of its total diameter against 9% in humans [25][6]. Although, in cats, there are no established reference values for the dimensions of the OB, it is accepted that felines have proportionally smaller OBs than carnivores [26][7]. It is also the first component of the basal portion of the rhinencephalon which, in turn, includes the structures of the basal telencephalon related to olfaction, the hippocampus, and associated structures [27][8]. Within the cranial cavity, the OB is located in the rostral cranial fossa, defined as the intracranial area extending from the optic canal and middle cranial fossa, caudally, and the cribriform plate, rostrally. The ethmoid fossa, separated from the nasal cavity by the cribriform plate, is an excavated continuation of the most rostral portion of the rostral cranial fossa. The rostral fossa receives not only the OB at the level of the ethmoid fossa, but also he olfactory peduncle and portions of the frontal lobe [28,29][9][10]. Internally, the OB can be filled by the so-called OB cavity or olfactory ventricle, an extension of the rostral horn of the lateral ventricles which, as such, is part of the cerebral ventricular system, allowing the free circulation of cerebrospinal fluid and participating in the rostral migratory stream [30,31][11][12]. Each OB is connected to the corresponding cerebral hemisphere through the olfactory peduncle, which has a short length and rapidly divides into the lateral, medial, and intermediate olfactory tracts or stria, each reaching a certain cortical territory [32][13]. The angle where the lateral and medial olfactory tracts separate rostrally delimits the so-called trigone or olfactory tubercle which, in turn, delimits the piriformis lobe cranially [25][6]. The rhinencephalon and, by extension, the OB and peduncles are separated from the medial and lateral portions of the corresponding hemispheres by the lateral and medial rhinal sulcus, which some authors also name as olfactory grooves, generating some inconsistency in the applied nomenclature. The lateral rhinal sulcus can be further divided into a rostral portion and a caudal portion [27][8]. The space that separates the OB from the rest of the brain was also tentatively referred to as the olfactory fissure [29][10].3. Histology of the Olfactory Bulb
Histologically, the OB consists of seven layers. From the most superficial, these are: [1] Olfactory nerves layer; [2] Glomerular layer; [3] External plexiform layer; [4] Mitral cell layer; [5] Internal plexiform layer; [6][14] Granular cell layer; and [7][15] Subependymal or periventricular layer [18][16]. These layers are strictly organized, facilitating the processing of olfactory information and its spatial encoding (Figure 1 and Figure 2).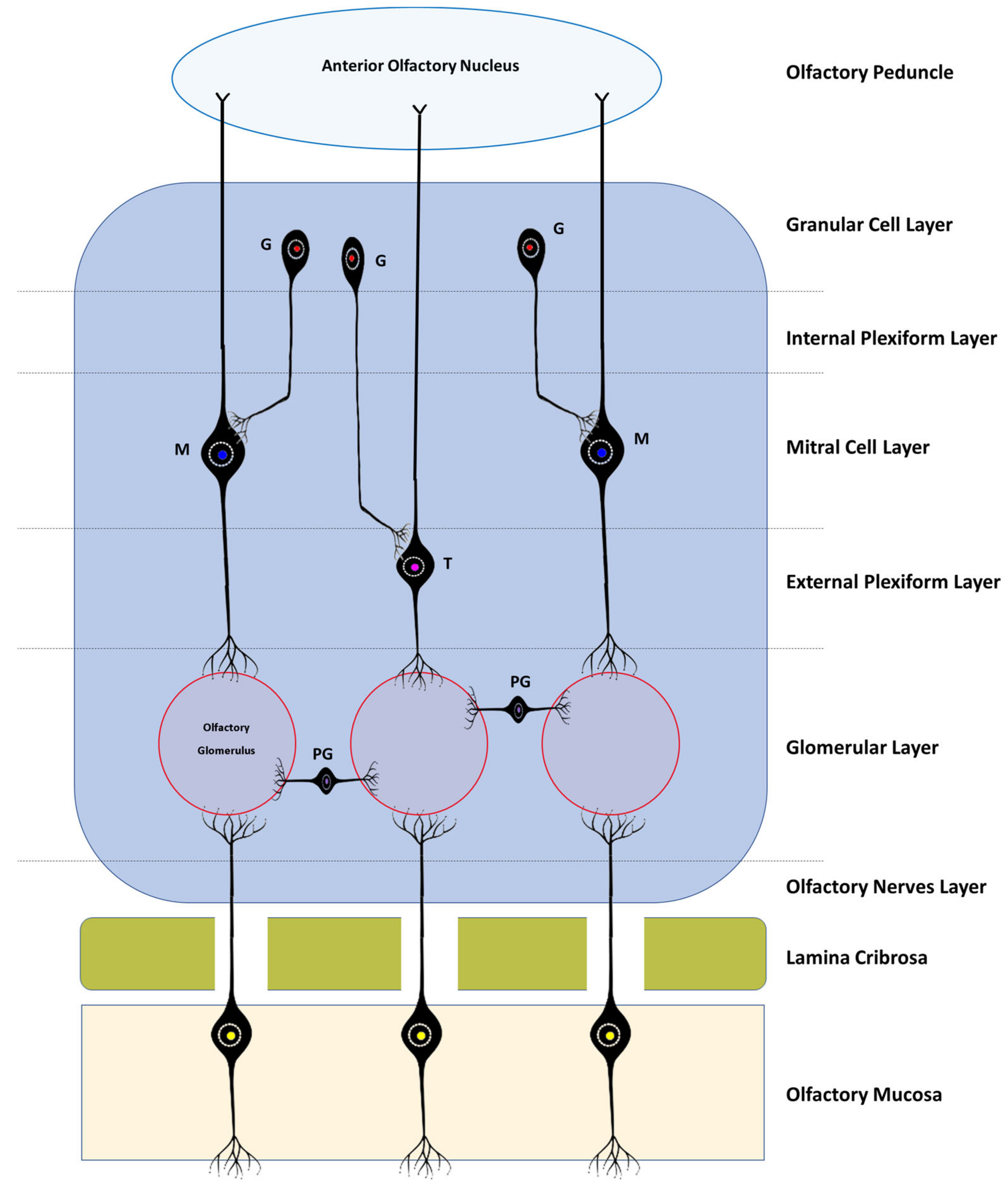
Figure 1. Simplified schematic representation of the histological layers of the OB, the interactions between the different bulbar cells, and the basic neural circuits in the OB. All the axons of the olfactory sensory cells project to a certain olfactory glomerulus depending on the type of receptor they have; PG—Periglomerular Cells; T—Tufted Cells; M—Mitral Cells; G—Granule cells.
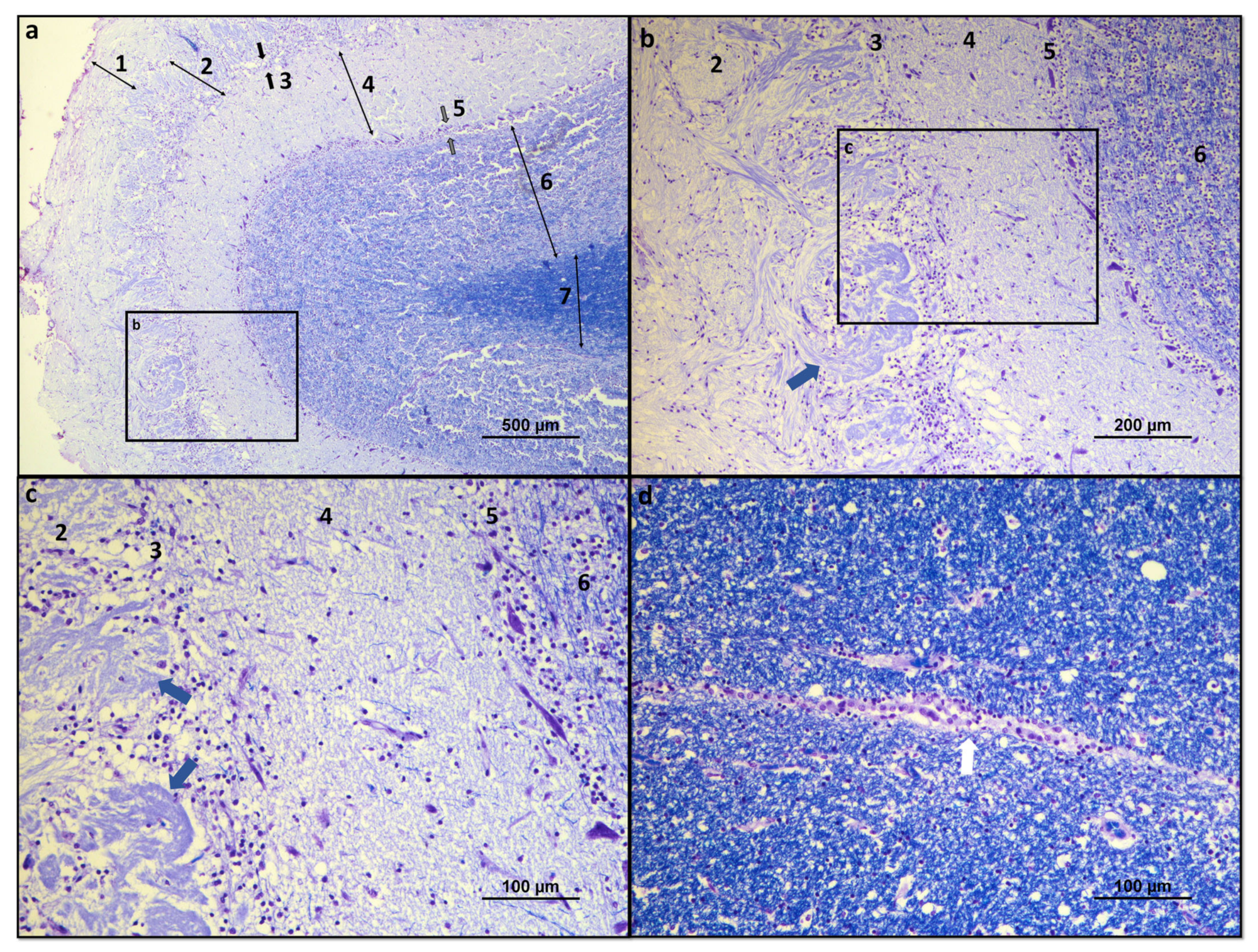
Figure 2. Histological overview of the dog OB: (a) Histological organization of the seven constituent layers of the olfactory OB: 1—olfactory nerves layer; 2—glomerular layer; 3—external plexiform layer (between black arrows); 4—mitral cell layer; 5—internal plexiform layer (between gray arrows); 6—granular cell layer; and 7—subependymal layer. (b) Structural details of the glomerular, external plexiform, mitral, internal plexiform, and granular layers. Olfactory glomerulus highlighted by blue arrow; (c) Structural details of the glomerular, external plexiform, mitral, internal plexiform, and granular layers. Olfactory glomeruli evidenced by blue arrows; (d) Structural details of the ependymal layer of the OB surrounding the olfactory ventricle, highlighted by a white arrow. Luxol fast blue and cresyl violet.
4. Olfactory Bulb and Diseases
Although, in human medicine, the approach of the OB as a relevant element in clinical evaluation has already been established for some time, in veterinary medicine, its importance is still largely neglected in the fields of clinical research. In humans, there is a well-recognized correlation between olfactory alterations and other clinical entities, and the transition volumes between normal and hypoplastic OB are well defined [60][22]. It is considered that up to 20% of the general human population suffer from some type of olfactory dysfunction such as hyposmia (deficits in the sense of smell) or anosmia (total absence of sense of smell) [51][23]. In companion animals, the prevalence of hyposmia is largely ignored, mostly due to the difficulty in unequivocally and quantitatively assessing olfactory acuity in these species [8][24], but in animals highly dependent on the sense of smell for their physiology and interaction with the environment, even moderate changes in olfactory acuity can be serious. At the same time, it is known that several neurodegenerative disorders such as Alzheimer’s disease or Parkinson’s disease are associated with olfactory alterations that appear as prodromal symptoms [51][23]. Furthermore, the relationship between a partial olfactory loss and an increased risk of depression is also known, with this clinical state being associated with a lower activation of all the structures involved in olfaction perception and with a decrease in the dimensions of the OB [61][25]. The volume of the OB can effectively be used as a predictor of the individual’s olfactory function and of the response to a treatment for primary or secondary olfactory alterations, being proportional to the individual’s olfactory sensitivity and to the volume and density of gray matter in the primary olfactory regions. Scenarios of post-traumatic or post-infectious anosmia or hyposmia translate into smaller volumes of the OB compared to healthy individuals [62][26] and, in congenital anosmia, the OB may not be identifiable by MRI or may be hypoplastic. Conversely, an increase in olfactory acuity associated with larger OB volumes has also been identified [51,60,63][22][23][27]. In dogs, several infectious diseases such as canine distemper and canine parainfluenza, endocrine diseases such as hyperadrenocorticism, hypothyroidism, or diabetes mellitus and even nasal tumors have already been identified as affecting the olfactory capacity, but it is thought that in these cases the alterations are associated with changes within the nasal cavity and in the olfactory neuroepithelium. Other cases such as granulomatous meningoencephalitis or head trauma may have a direct influence on the brain regions related to olfactory processing, but it is not known at what level the OB may be involved [11][28]. In humans, olfactory function decreases with age due to various changes ranging from cumulative lesions in the olfactory epithelium, structural changes in the nasal cavity, and brain modifications secondary to the onset of neurodegenerative diseases [64][29]. Similar alterations can be identified in older dogs, which show both a significant decrease in the number of cilia and olfactory receptors in the olfactory neuroepithelium and senile changes in the OB such as cerebrovascular amyloidosis [65][30]. Some studies establish a relationship between the onset of neurodegenerative diseases and a decrease in OB volume in humans, with consequent impairment of the entire olfactory process [66,67][31][32]. Although brain structural changes have already been well identified in dogs suffering from cognitive disfunction [68][33], there is a lack of studies in companion animals that allow us to create a link between the decrease in OB volume and the establishment of such clinical scenarios. Hyposmia secondary to disease or trauma is normally reversible; the same not being observed in the case of changes associated with aging and neurodegenerative disorders. Another common finding in older individuals and in certain pathologies, and which has already been identified both in humans and in dogs and cats, is the general enlargement of the ventricular system in normal patients and associated with disease processes [69][34]. In some individuals, the olfactory recess of the lateral ventricle, which is usually an empty virtual space, may become distended with cerebrospinal fluid (CSF). This OB ventricular dilatation has not been associated with ageing in geriatric cats [69,70][34][35] despite ageing being associated with generalized ventriculomegaly. In humans, generalized ventriculomegaly associated with an increase in the volume and pressure of the CSF and consequent compression of the adjacent brain tissue is often secondary to changes in fluid drainage associated with a variety of conditions including uncontrolled diabetes mellitus, [71][36], or as an ex-vacuo effect due to atrophy and loss of neuronal tissue in senility [72][37]. Currently, there are no studies suggesting that dilation of the olfactory recesses in cats and dogs are markers for senility or systemic disease, although pathologic obstruction of ventricular drainage in cases with forebrain neoplasia has been described as causing dilation of the olfactory recesses in companion animals [58][38].References
- Shepherd, G.M.; Greer, C.A. Olfactory Bulb; Oxford University Press: Oxford, UK, 1998.
- Lodovichi, C. Topographic organization in the olfactory bulb. Cell Tissue Res. 2021, 383, 457–472.
- Poncelet, G.; Shimeld, S.M. The evolutionary origins of the vertebrate olfactory system. Open Biol. 2020, 10, 200330.
- Jurkowski, M.P.; Bettio, L.K.; Woo, E.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the hippocampus and the SVZ: Adult neurogenesis throughout the brain. Front. Cell. Neurosci. 2020, 14, 576444.
- Curtis, M.A.; Monzo, H.J.; Faull, R.L. The rostral migratory stream and olfactory system: Smell, disease and slippery cells. Prog. Brain Res. 2009, 175, 33–42.
- Kavoi, B.M.; Jameela, H. Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int. J. Morphol. 2011, 29, 939–946.
- Van Valkenburgh, B.; Pang, B.; Bird, D.; Curtis, A.; Yee, K.; Wysocki, C.; Craven, B.A. Respiratory and olfactory turbinals in feliform and caniform carnivorans: The influence of snout length. Anat. Rec. 2014, 297, 2065–2079.
- Veterinaria, N.A. International Committee on Veterinary Gross Anatomical Nomenclature (ICVGAN); Editorial Committee: Hannover, Germany, 2017.
- Sokołowski, W.; Barszcz, K.; Kupczyńska, M.; Czopowicz, M.; Czubaj, N.; Kinda, W.; Kiełbowicz, Z. Morphometry and morphology of rostral cranial fossa in brachycephalic dogs—CT studies. PLoS ONE 2020, 15, e0240091.
- Hussein, A.K. Nomenclature and descriptive anatomy of the olfactory bulb fissure and definition of the olfactory bulb dimensions in dogs using in vivo mri. Int. J. Adv. Res. 2019, 7, 1120–1125.
- Malik, S.Z.; Lewis, M.; Isaacs, A.; Haskins, M.; Van Winkle, T.; Vite, C.H.; Watson, D.J. Identification of the Rostral Migratory Stream in the Canine and Feline Brain. PLoS ONE 2012, 7, e36016.
- Smitka, M.; Abolmaali, N.; Witt, M.; Gerber, J.; Neuhuber, W.; Buschhueter, D.; Puschmann, S.; Hummel, T. Olfactory bulb ventricles as a frequent finding in magnetic resonance imaging studies of the olfactory system. Neuroscience 2009, 162, 482–485.
- Andrews, E.F.; Pascalau, R.; Horowitz, A.; Lawrence, G.M.; Johnson, P.J. Extensive Connections of the Canine Olfactory Pathway Revealed by Tractography and Dissection. J. Neurosci. 2022, 42, 6392–6407.
- Mohamed, S.A.; Salem, H.F.; Ebraheim, L.L.; El-Behery, E.I.; El-Naseery, N.I. Species Specific Structural Differences of the Main Olfactory Bulbs in Dog (Canis Familiaris) and Goat (Capra Hircus). Zagazig Veter. J. 2020, 48, 67–78.
- Hughes, N.K.; Price, C.; Banks, P. Predators Are Attracted to the Olfactory Signals of Prey. PLoS ONE 2010, 5, e13114.
- Wei, Q.-G.; Zhang, H.-H.; Guo, B.-R. Histological structure difference of dog’s olfactory bulb between different age and sex. Zool. Res. 2008, 29, 537–545.
- Enagayama, S.; Homma, R.; Imamura, F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits 2014, 8, 98.
- Imamura, F.; Ito, A.; LaFever, B.J. Subpopulations of Projection Neurons in the Olfactory Bulb. Front. Neural Circuits 2020, 14, 561822.
- Takahashi, H.; Yoshihara, S.; Tsuboi, A. The Functional Role of Olfactory Bulb Granule Cell Subtypes Derived From Embryonic and Postnatal Neurogenesis. Front. Mol. Neurosci. 2018, 11, 229.
- Arruda, D.; Publio, R.; Roque, A.C. The Periglomerular Cell of the Olfactory Bulb and its Role in Controlling Mitral Cell Spiking: A Computational Model. PLoS ONE 2013, 8, e56148.
- Tufo, C.; Poopalasundaram, S.; Dorrego-Rivas, A.; Ford, M.C.; Graham, A.; Grubb, M.S. Development of the mammalian main olfactory bulb. Development 2022, 149, dev200210.
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.; Witt, M.; Abolmaali, N.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. Neuroimage 2008, 42, 498–502.
- Desser, D.; Assunção, F.; Yan, X.; Alves, V.; Fernandes, H.M.; Hummel, T. Automatic segmentation of the olfactory bulb. Brain Sci. 2021, 11, 1141.
- Polgár, Z.; Kinnunen, M.; Újváry, D.; Miklósi; Gácsi, M. A Test of Canine Olfactory Capacity: Comparing Various Dog Breeds and Wolves in a Natural Detection Task. PLoS ONE 2016, 11, e0154087.
- Croy, I.; Hummel, T. Olfaction as a marker for depression. J. Neurol. 2017, 264, 631–638.
- Frosolini, A.; Parrino, D.; Fabbris, C.; Fantin, F.; Inches, I.; Invitto, S.; Spinato, G.; De Filippis, C. Magnetic Resonance Imaging Confirmed Olfactory Bulb Reduction in Long COVID-19: Literature Review and Case Series. Brain Sci. 2022, 12, 430.
- Huart, C.; Rombaux, P.; Hummel, T. Plasticity of the Human Olfactory System: The Olfactory Bulb. Molecules 2013, 18, 11586–11600.
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the nose doesn’t know: Canine olfactory function associated with health, management, and potential links to microbiota. Front. Vet. Sci. 2018, 56, 1–18.
- Doty, R.; Kamath, V. The influences of age on olfaction: A review. Front Psychol. 2014, 5, 20.
- Hirai, T.; Kojima, S.; Shimada, A.; Umemura, T.; Sakai, M.; Itakurat, C. Age-related changes in the olfactory system of dogs. Neuropathol. Appl. Neurobiol. 1996, 22, 531–539.
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24.
- Tremblay, C.; Mei, J.; Frasnelli, J. Olfactory bulb surroundings can help to distinguish Parkinson’s disease from non-parkinsonian olfactory dysfunction. NeuroImage Clin. 2020, 28, 102457.
- Dewey, C.W.; Rishniw, M.; Johnson, P.J.; Platt, S.; Robinson, K.; Sackman, J.; O’Donnell, M. Canine cognitive dysfunction patients have reduced total hippocampal volume compared with aging control dogs: A comparative magnetic resonance imaging study. Open Veter. J. 2020, 10, 438–442.
- Babicsak, V.R.; Klein, A.V.; Tsunemi, M.H.; Vulcano, L.C. Age-related changes of the cerebral ventricles of healthy domestic cats. Pesqui. Veterinária Bras. 2018, 38, 1935–1941.
- McGregor, O.; Genain, M.; Williams, T.L.; Alves, L. Prevalence and clinical correlations of olfactory recess dilatation in MRI studies of the feline brain. In Veterinary Radiology & Ultrasound; Wiley Online Library: Hoboken, NJ, USA, 2023.
- Yamada, S.; Ishikawa, M.; Nozaki, K. Exploring mechanisms of ventricular enlargement in idiopathic normal pressure hydrocephalus: A role of cerebrospinal fluid dynamics and motile cilia. Fluids Barriers CNS 2021, 18, 1–11.
- Todd, K.L.; Brighton, T.; Norton, E.S.; Schick, S.; Elkins, W.; Pletnikova, O.; Fortinsky, R.H.; Troncoso, J.C.; Molfese, P.J.; Resnick, S.M.; et al. Ventricular and Periventricular Anomalies in the Aging and Cognitively Impaired Brain. Front. Aging Neurosci. 2018, 9, 445.
- Brunjes, P.C. The mouse olfactory peduncle. 2.The anterior limb of the anterior commissure. Front. Neuroanat. 2013, 6, 51.
More
