Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Wathiq Mansoor.
Parkinson’s disease (PD) is a devastating neurological disease that cannot be identified with traditional plasma experiments, necessitating the development of a faster, less expensive diagnostic instrument. Due to the difficulty of quantifying PD in the past, doctors have tended to focus on some signs while ignoring others, primarily relying on an intuitive assessment scale because of the disease’s characteristics, which include loss of motor control and speech that can be utilized to detect and diagnose this disease. It is an illness that impacts both motion and non-motion functions. It takes years to develop and has a wide range of clinical symptoms and prognoses.
- Parkinson’s disease
- computational intelligence
- deep learning
- diagnosis
- machine learning
1. Introduction
PD is a chronic movement illness that impacts the whole body. Around the world, 7 to 10 million people are suffering from PD [1]. By 2030, there will be 8.7 million to 9.3 million instances of Parkinson’s disease worldwide, according to the most current forecast from the European Parkinson’s Disease Association [2]. The impact of PD on voice patterns is a symptom that has received little attention. In this disease, the range of speech phonation motions (lips, tongue, and jaw) is limited (hypokinetic), and, as a result, vowels become centered, i.e., formants with particular rates have lower rates, whereas formants with low rates have higher frequency ranges [3]. In addition to movement abnormalities, Parkinson’s is associated with non-motor symptoms, such as restlessness at night, tiredness, and so on. It was recently discovered that almost all patients have non-motor symptoms in addition to the traditional motor symptoms, which vary depending on the severity of the disease [4]. Tremor, stiffness, postural instability, and bradykinesia are all motor signs of PD caused by insufficient dopamine signaling caused by dopamine-producing neurons being destroyed in the substantia nigra portion of the brain [5].
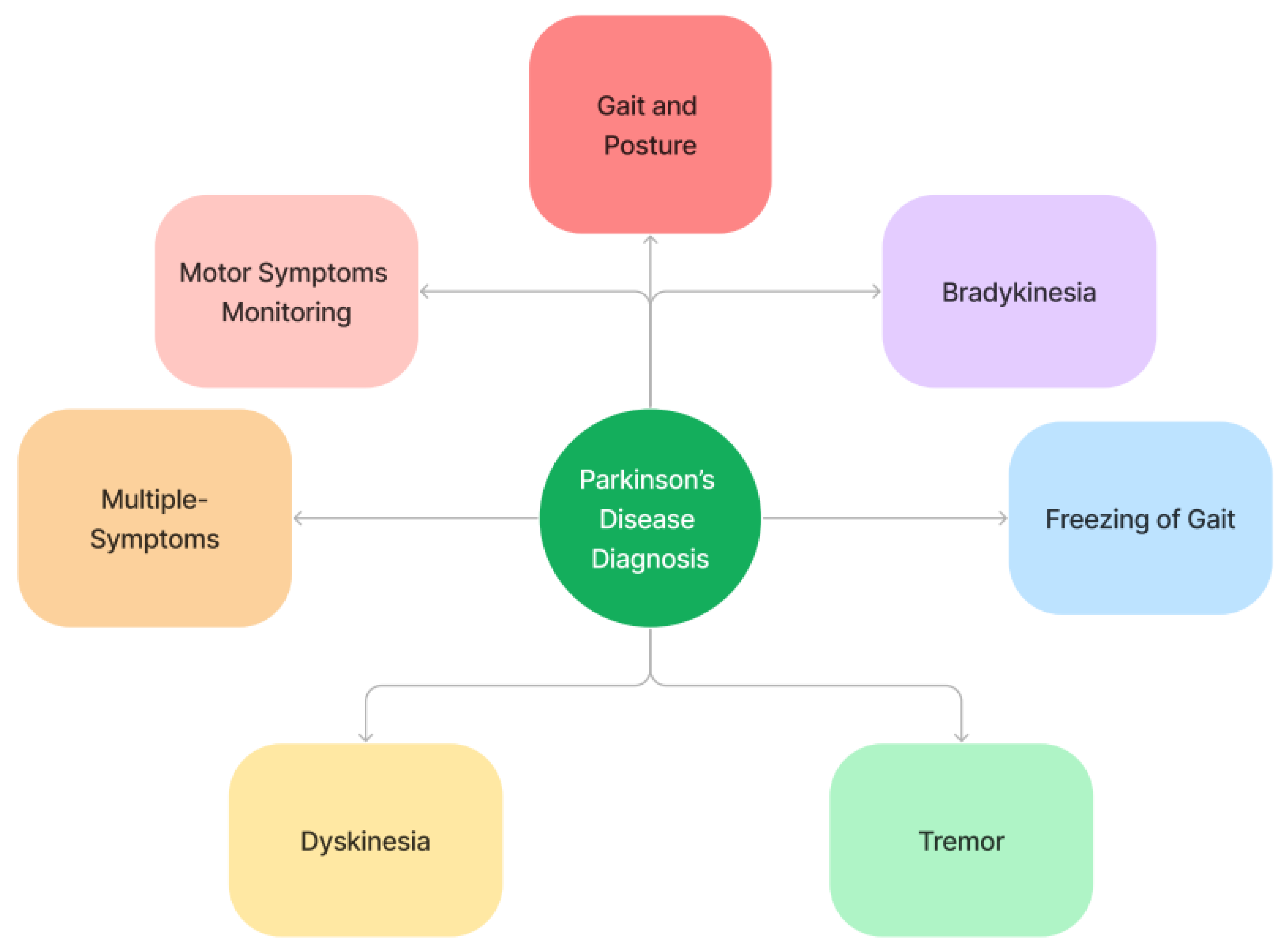
2. Parkinson’s Disease Diagnosis
A thorough history and physical examination should be part of the differential diagnosis of Parkinson’s disease (PD). Referring challenging or dubious cases to a mobility disorder specialist for additional assessment is recommended. Since there are no conclusive tests to confirm the diagnosis of PD, a clinical diagnosis must be made by a physician after considering the patient’s past medical history, evaluating their symptoms, and ruling out other conditions, such as multiple-system atrophy, DLB illness, and fundamental movements. Figure 41 illustrates an overview of Parkinson’s disease diagnosis.
Figure 41.
Parkinson’s disease diagnosis—overview.
2.1. Motor Symptoms Monitoring
The key motor aspects of Parkinson’s are akinesia (lack of action, trouble beginning motions) and bradykinesia (slow movements). Rigidity is linked to the patient’s sensation of stiffness, and clinicians can quantify rigidity by looking at a muscle’s resistance to passive stretching. The presence of a subset of motor symptoms is required for the clinical diagnosis of parkinsonism; hence, the diagnostic approach accommodates patients with varying motor health statuses. An individual with the disease can “freeze up” and would be unable to walk for a brief amount of time. Furthermore, categorization into the three primary severity groups (mild, moderate, and severe) was further separated into dichotomous issues, in which binary classifiers outperform and pick various sets of non-motor indicators [16][6]. Loss of scent is one of the non-motor indications and symptoms of PD, including anosmia, nerve damage, urinary incontinence problems, bowel problems, depression and anxiety, sleep issues (insomnia) leading to daytime sleepiness, cognitive issues, psychotic episodes, fantasies, and depression. The majority of current therapeutic practice is devoted to the pharmacological treatment of motor complaints. It takes years to develop and has a wide range of clinical symptoms and prognoses. There is no single scenario or course that can depict the whole range of motor and cognitive symptoms that Parkinson’s patients encounter. Moderate motor-predominant PD strikes people while they are young (in their 50 s or 60 s). Even though the signs are more noticeable than in the light engine version, these people can nevertheless work and live busy lives. People may have moments when the medicine effectively cures motor symptoms, but they may have levodopa-induced dyskinesias as the condition worsens. These people can suffer off time towards the conclusion of the dosage when the motor symptoms reappear. Patients’ sickness advances over time to a more severe stage, yet they normally survive for a long period before becoming disabled [17][7].2.1.1. Gait and Posture
With no uniformity in the study or justification for why a trait was included in the illness assortment, gait features in the literature greatly vary. Previous research has experienced several problems, such as people who had more serious conditions, a limited sample size, and a lack of real-world data to identify the ideal gait features. As a result, the findings are less generalizable, valid, and applicable. As a result, substantial trials for Parkinson’s classification in patients with less severe illness employing a set of clearly interpretable and quantifiable gait features are required. Step size, speed, breadth, step time, swing time, posture time, and their corresponding variability and asymmetry are the distinguishing features of gait. Deficiencies are a prevalent symptom of parkinsonism that develop early and progress over time. The current method for defining gait impairment is a univariate method, which makes understanding the role of various gait parameters challenging. Therefore, a top priority to increase the use of gait traits as a technique to enhance illness diagnosis and management is to identify the best combination of gait attributes to detect PD more effectively [19][8]. For research purposes, the PhysioNet repository provides access to a large database of physiological parameters. In this deep-learning-based study, by using two separate symptoms linked with PD, gait and speech loss, a neural network is utilized to identify the disease. Patients with significant gait problems are at risk of falling and losing their functional independence. Each subject’s 16 recorded sensor readings are turned into a spectrogram picture, which portrays a pattern by graphing the sensors’ fluctuating signal levels [20][9]. Ground reaction force is the pressure that the ground applies to a body that is in contact with it. VGRF is the strongest factor of the ground reaction force during walking, producing forces that are larger than one body weight (BW) per step. For gait analysis and characterization, the VGRF signals contain significant information. As a result, this method easily might be applied to other gait clinical trials in a clinical context. Rather than storing discriminative similarities between Parkinson’s and control gaits, the algorithm in this case might save specific subject gait features. For future research, going into the DNN layers and analyzing what they have learned might be interesting. This type of research would help us learn more about PD gait and its characteristics [21][10]. In the early phases of the syndrome, gait is characterized by reduced leg speed and amplitude, as well as reduced arm swing [22][11]. Patients in the Fox Insight study said that jogging or mowing the lawn could trigger tremors, and phone analyses of gait metrics generated from this raw sensor information were also shown to be erroneous for people living in congested locations. [23,24][12][13].2.1.2. Bradykinesia
Bradykinesia is characterized by a loss of conscious motor function, as well as sluggish or frozen motions. It is most usually a sign of PD or a drug adverse effect. It is one of the most obvious signs that physicians check for when diagnosing PD [30][14]. It frequently develops in the initial stages of the disease [31][15] and is specific to the disease of the basal ganglia [32][16]. Actions may be delayed (bradykinesia), decreased (hypokinesia), or altogether abolished (akinesia) depending on the degree. Facial muscular bradykinesia is another kind of dyskinesia that affects the face. During the assessment phase, while various body segments are at varied levels of repose, the consistency of rest tremors is assessed using a single score for all tremors [33][17]. Overall, medicine improved bradykinesia and tremor measurements, whereas treatment worsened non-motor indicators. Dopaminergic drugs have many positive benefits, such as reducing bradykinesia and stiffness, but they also have some bad impacts, such as hypersomnolence and impulsive control issues [34][18]. By utilizing bradykinesia and rigidity derived from recent clinical and pathological studies, the positive predictive value of diagnosis can be raised to above 95% [34][18].2.1.3. Freezing of Gait
FOG is a kind of akinesia characterized by the inability to begin or maintain movement. Motion blockages are a typical sign of Parkinson’s, and they can affect any of the body’s extremities, as well as the face. According to its definition, it refers to a condition where there is a brief, episodic disappearance or considerable diminution of forward movement of the feet. The sensation usually lasts for a few seconds after it arises and then vanishes. It is a typical reason for people to trip and fall [30][14]. It is a common gait problem in people with advanced PD. FoG episodes have been associated with falls, which disrupt daily activities and reduce the quality of life. For patients with advanced diseases, it is a prevalent gait condition. Falls have been linked to episodes, which disrupt everyday activities and lower quality of life. It is usually resistant to pharmacologic therapy, requiring the use of effective non-pharmacologic treatments. It is the sort of gait impairment that is common in Parkinson’s patients. It causes patients to feel as if their feet are stuck to the ground and that they are briefly unable to re-establish gait. These attacks might last for anything from a few seconds to a minute. It can emerge everywhere, although it is particularly common during turns, before gait begins, in small spaces such as doors, and in stressful situations.Another significant challenge is recognizing and analyzing PD-related movement patterns, such as gait start and gait freezing, which are all typical illness markers. According to one medical study, 90% of people with the disease exhibit vocal impairment, making it critical to analyze speech data to distinguish healthy people from those with the disease. Various symptoms are important in the diagnosis, treatment, and therapy of PD. Three symptoms are present: difficulty starting or maintaining walking, complete immobility, and staggering with swift steps [37][19]. About half of PD patients experience this extremely debilitating symptom in the latter stages of the illness [38][20].2.1.4. Tremor
Tremor is described as a progressive loss of muscle control that causes quivering (uncontrollable shaking) in numerous body parts [46][21]. Several characteristics are crucial in determining the identification, treatment, and therapy of PD. Tremor is a limb twitching that is involuntary and oscillatory. Rigidity in motion is induced by increased muscular tone. Loss of balance and unexpected falls are caused by postural instability. Because of these mobility issues, people with PD show different gait characteristics from healthy people. One of the most significant components of disease management appears to be tremor diagnosis and classification. Body posture detection is also essential because automated systems only based on their base frequency have difficulties distinguishing the two. Motor assessment of PD is the evaluation of tremors combined with bradykinesia, as well as evaluations of tremors in the mouth, jaw, bottom lip, arm, or leg [143][22]. The UPDRS scale is an unreliable method for assessing and discriminating tremor severity, and clinical examination and tremor incidence determination require the presence of at least one neurologist. It is critical to create a method or piece of equipment that can assess the intensity in Parkinson’s patients. Frequency and intensity of tremors are two main parameters that have been cited in most previous publications for objective categorization of not only Parkinson’s tremors, but also other forms of tremors [47][23].Their gait is characterized by a forward flex posture, quick shuffling, shorter step lengths, and prolonged support intervals, among other characteristics [48][24].2.1.5. Dyskinesia
Dyskinesia is the incapacity of people with PD to control their muscle movements. Twitches, jerks, twisting, and writhing are examples of such movements. Dyskinesia can affect the arms, legs, and chest, among other body regions. There are various sorts of movements, and the timing and frequency with which they emerge vary from person to person with Parkinson’s disease. Dyskinesia can last for the majority of the day in some people. Others may only notice it after they have taken their prescription, or right before their next dose is due. When levodopa levels in the bloodstream are very low and dopamine levels in the brain are at their peak, people with Parkinson’s disease may suffer this adverse effect. The neurotransmitter dopamine is created in the brain. PD symptoms appear when dopamine is very low. Even though the symptoms are more noticeable than in the mild motor-predominant version, these people can nevertheless work and live busy lives. People may have moments when the drugs successfully cure motor symptoms (on-time), but they may have levodopa-induced dyskinesias as the disease progresses [17][7]. More UPDRS components will be compiled, and the addition of other four Parkinson’s characteristics, such as rigidity or dyskinesia, will be explored as enhancements to the current study [47][23]. A previous study made efforts to tackle the use of wearable sensors to count distinct types of PD symptoms, only using bimodal distribution or unimodal sensors [52][25].2.2. Speech Monitoring
Dysarthria is a symptom that significantly differs between cohorts. This kind of heterogeneity could be attributed to subject-level and task-related cognitive factors. A crucial classification difficulty is the proper representation of voice and speech data for PD diagnosis. To use these resources for PWP (People with Parkinson’s), accurate clinical monitoring tools must be used. According to research, around 90% of PWP suffer voice impairment and speaking issues. Loudness, decrease, breathiness, roughness, and increased vocal tremors are the five major clinical symptoms of dysphonia in humans. Looking at the frequency of time in speech recordings can provide all of these characteristics [53][26]. Abayomi et al. [54][27] indicated that a quick and easy data augmentation method based on spline and pchip interpolation has been shown to be successful in the diagnosis of PD, particularly if the sample is of voice impairment. Dopamine is a neurotransmitter that allows the brain to effectively communicate when it comes to managing feelings, behaviors, consciousness, physical movement, and speaking ability. Analyzing and classifying patients’ speech signals is thought to be a way to diagnose PD early by distinguishing characteristics and aspects of their voices [55][28]. The goal of the study was to see if speech difficulties may be detected in the early stages of PD, before the traditional symptoms appear, and if those at risk of the condition can be identified from the general population using acoustic and classification analyses. In it, speech articulator motions (lips, tongue, and jaw) are limited in range (hypokinetic), and vowels become centralized; i.e., formants with high frequencies are likely to have a lower frequency, whereas consonants with lower frequencies tend to have higher frequencies [56][29]. Speech monitoring and repair assessments significantly separated the groups and were linked to linguistic performance tests [57][30]. One typical PD characteristic is hypokinetic dysarthria, a combination of neuromuscular speech disorders that impair understanding, self-image, and productivity. There has been no study that has looked at this idea while controlling both task-related and specific topic cognitive factors, while avoiding previous flaws. Monolith, monologs, and inappropriate pauses are all prevalent deficiencies in the prosody area. Disturbances in the basic frequency of vocal-fold vibration and language latencies cause such changes. Speech signals can be used to diagnose the disease. Affected individuals have a lot of speaking issues. Reduced speech intensity, variation in frequency components, hoarseness in voice, and inconsistency in speech articulation (hypokinetic dysarthria) were among the speech impairments. Due to the presence of non-stationary and discontinuity in the speech signal, extracting and classifying speech features has always been a difficult problem [58][31]. An important classification difficulty is the appropriate interpretation of voice and speech data to identify PD. The depletion of neurotransmitters, notably dopamine, results in a variety of symptoms, including speech, vision, mobility, urine issues, weight loss, sadness, anxiety, panic attacks, sleep abnormalities, and so on. Dysphonia, hypophonia, monotonic, and dysarthria are some of the vocal and speech problems that PWP suffer from [59][32]. The most common symptoms are dysphonia, which is present in over 90% of patients, and gait unpredictability, which is a distinguishing criterion for the development of this condition. As a result, enhanced speech signal processing technology for PD symptoms has sparked a lot of interest [60][33].2.3. Handwriting Analysis
The complicated process of handwriting requires intellectual, visual, and fine motor skills. Handwriting difficulty is still one of the earliest symptoms that lead individuals to seek medical attention, despite not being explicitly included in the diagnostic criteria for PD. The majority of PD sufferers display faulty handwriting. Recent research has revealed that auditory cueing has a good impact on handwriting proficiency, but visual feedback has conflicting results. The degree to which a handwriting assignment is beneficial can be determined by comparing its fluency with and without the provision of feedback. Since the development of kinematic analysis via the use of digitized tablets, research on handwriting in PD has experienced a significant revolution. The traditional PD handwriting anomaly known as micrographia is now included in the spectrum of “PD dysgraphia”.2.4. Face Video Analysis
There is a lot of interest in utilizing machine learning to help with disease diagnosis, and the present results are promising—often, the claimed accuracy percentage only using speech or accelerometer data is in the mid-90s [63][34]. However, because the experimental methodology involves distinguishing between (possibly erroneously) diagnosed PD patients and healthy controls, these findings should not be taken at face value (HCs). In the realm of smart recognition, the ability to identify individual attributes is a global security problem [64][35]. Ref. [65][36] suggested an LSTM model that will aid in providing patients with more thorough care and assisting medical professionals in better comprehending the dynamics of the disease in real time. Additionally, it aimed to ease the burden on doctors’ recurrent patient diagnoses and the issue of enrolling patients who have mobility issues. Various algorithms have been created in recent years to address the security issue, but there is still a need for quick and efficient biometric recognition. Biometric recognition is the process of automatically recognizing an individual’s qualities based on anatomical or behavioral features. Extrinsic biometric features and intrinsic biometric features are the two types of biometric recognition approaches [30][14]. When compared to intrinsic traits, extrinsic features are more visible and have more negative aspects. The retinal surface, for example, is influenced by the high intensity of light used to extract iris characteristics [31][15]. Face identification accuracy is further impacted by brightness differences, facial style, blood vessel obstruction, and position [32][16]. Face recognition systems [38][20], motion detection systems [66][37], and other applications are among them.2.5. Brain Imaging
Cho et al. [19][8] used an advanced computer-based method to analyze brain MR imaging of subject areas with suspicious characteristics of Parkinson’s to contribute to individual diagnosis, based on the idea that diseases linked with systematic changes in brain MR scanning are too uncertain to be noticed by visual inspection. This is a very cost-effective procedure that is supposed to supplement, not substitute, current treatments for obtaining an early and accurate diagnosis of the disease, because existing MR imaging data is repurposed with modern information processing. They presented the study as initial evidence for the feasibility of performing SVM personalized classification of DTI data of Parkinson’s, indicating the need for further prospective and more extensive follow-up studies. Although brain MR imaging is frequently used in diagnostic procedures, it is mostly used to rule out other conditions, such as regular hydrocephalus and chronic subdural hemorrhage.2.6. Using Multimedia Approaches
A lot of studies [68,69,70][38][39][40] have built PD detection mechanisms using various types of datasets. The data were evaluated, and information was extracted using feature identification techniques (image, text, audio, and video). In many studies, simple body sensing data (text), speech data (audio), image features (pictures), and motion sensors are all examples of simple body sensor values. These studies have not focused on building machine learning and deep learning techniques for managing inter datasets.2.7. Stage-Wise Prediction of Parkinson
In the beginning, the person only experiences minor symptoms that do not affect daily life. Shen et al. [179][41] developed a methodology for sparse feature learning in PD early detection. During stage 1, only one side of the body has tremors and other movement symptoms [180][42]. Balance is not harmed in stage 2. Furthermore, the person’s posture may start to shift, and walking difficulties may start to emerge or worsen [181][43]. Loss of balance is the defining characteristic of the middle stage/stage 3. Motor symptoms become worse. Though physically capable of living independently, the person’s everyday activities are now restricted in a functional sense. Stage 4 symptoms are completely formed and quite incapacitating. The person can still stand and walk unaided, but, for safety reasons, they may need to use a cane or walker [180][42]. The most advanced stage of Parkinson’s disease is stage 5. Advanced leg stiffness can also result in freezing when standing, which makes it impossible to move or stand [181][43].3. Datasets for Parkinson’s Disease Diagnosis
Another obvious sign of PD is a decline in handwriting skills, which are commonly observed in most PD patients but are not considered diagnostic criteria for the disease [60][33]. One of the three widely used PD handwriting datasets, the PaHaW dataset [72][44], HandPD [7][45], or NewHandPD [64][35], were used in thirteen experiments on deep learning algorithms that sought to diagnose PD using handwritten drawings. The spiral sketching test is one of the tests included in all three databases, and is one of the drawing and writing tests present in all three databases. Dataset can also be created by assessing equal number of people with PD and HC as done in [154][46]. For each modality (such as MRI, EEG, voice, etc.), DL studies may utilize a distinct dataset to develop their models. For instance, instead of using the public dataset, PPMI, MRI studies may choose to use a private dataset. As a result, it could be challenging to compare the effectiveness of two DL models that were trained using different datasets [73][47]. No restrictions on drop-out (or bias investigation), report of inclusion/exclusion criteria, or relationships between prodromal markers were found in [74][48].4. Machine Learning and Deep Learning Models for Parkinson’s Disease Diagnosis
4.1. Need for Machine Learning and Deep Learning Models for Parkinson’s Disease Diagnosis
To maximize ML’s generalization capabilities in neuroscience, many sorts of validation processes will be required. Testing different and new data on the same training model at the same time is the next step in attaining the best diagnosis accuracy. ML approaches have been widely utilized to predict PD across a variety of datasets. Furthermore, picking features for a new training model each time prevents the model from being automated and put into practice. Finally, ML algorithms that allow for incremental data updates and re-learning must be researched further. When large-scale labeled datasets are available, CNNs have already shown tremendous achievements in terms of navigating classification challenges in recent years [75][49]. The combination of ML models with feature selection methods enables the evaluation of the relative value of characteristics in a wide feature space to choose the most distinguishing ones, which is difficult to manually do [76][50]. The accuracy rate of deep learning techniques improves as the size of the dataset grows. However, to reproduce absolute speech data features from raw datasets, appropriate speech-processing algorithms must be devised. Traditional DL algorithms must also be updated for changing auditory information [71][51]. Old PD diagnosis requires a large number of observations in everyday activities, fine motor skills, and other brain features; however, this method is inadequate for detecting the disease early. ML and AI approaches have a lot of potential for categorization, according to previous research, and the classification system increases the validity and consistency of the diagnosis, as well as minimizing errors and boosting the efficacy of the process [26][52].4.2. Machine Learning Techniques
4.2.1. Artificial Neural Network
ANNs are often depicted as networks of interconnected “neurons” capable of calculating values from inputs/outputs and of pattern recognition and machine learning. Bind et al. [16][6] suggested using an ANN to diagnose PD in a dataset of ill and healthy patients using a boosting committee machine. Neural networks with backpropagation filtering techniques employ a majority voting scheme. Indeed, 75.4% of the 195 samples tested positive for PD, with the rest being healthy. Sachdev and Kim [78][53] studied the gait characteristics of 93 PD patients and 73 healthy adults. The disease has been discovered to utilize multiple biomarkers, which have been used in various investigations to identify the onset of the condition and its associated issues.AM García [18][54] developed the Multi-Layer Perceptron with a back-propagation learning method and RBF to forecast PDs [80][55]. The model for this module is based on ANN, another DL paradigm. It is divided into four layers, each of which has 64, 32, and 16 neurons. The dataset is supplied as a CSV file, from which it learns the non-linear trends in signal values and teaches itself. Tensor flow and Kera are also used in its development. When the issue is properly categorized or tiered into hierarchical levels, a tree functions well in diagnostic testing. Classification results are often good if sufficient data is provided to train ANNs.4.2.2. Naïve Bayes
The NB Classifier is a probabilistic classifier that assumes the presence of one class characteristic that is unrelated to the presence of other factors [86][56]. For writing tasks and spiral drawing, they employ an NB algorithm, with different metrics for each challenge. With an accuracy of 83.2%, the fourth task has the greatest classification accuracy [46,87][21][57]. It is sometimes referred to as a probabilistic predictor because of the probabilistic relationship between the category and the attributes. It does not have a deterministic relationship and is very extensible. The training is carried out in linear time by calculating a closed-form expression, as opposed to the iterative approximation used by many other classifiers. It has shown the lowest accuracy (71.79%) in detecting the presence of PD [88][58]. With more training data, the classification accuracy of this algorithm will drop. According to the best training data chosen by the PSO algorithm, the greatest accuracy in the potential classification for PD diagnosis may be reached with only eight training data. Researchers have proposed a new PD diagnostic model based on a PSO algorithm, and a combination of Naive Bayesian Classification and other algorithms. The PSO approach was used to select the best training data for Naive Bayesian Classification. By picking the best training data and avoiding those that produce a drop and decline in classification accuracy, the algorithm achieved a classification accuracy and PD diagnosis of 97.95%. This classification accuracy demonstrates the proposed method’s advantage over existing disease diagnostic models.4.2.3. Decision Tree
There are two research phases in the data mining module. The first involves applying association rule mining algorithms to analyze the patient’s status using raw patient data, therapy, patient profiles, and other publicly available data as part of the rule discovery process. The progress of automated symptom detection based on patient time series is the second step. This forecast is based on decision trees, and it aims to be more accurate than the prior study [6][59]. The fact that decision trees express rules is one of the most appealing features. Humans can easily grasp rules when they are written down. Nilashi et al. [95][60] attained the highest accuracy of 82% utilizing a decision tree on gyroscope data acquired with the Shimmer. To classify it, they employed the J48 decision tree included with the Weka software. Scholars from all across the world are interested in how medical datasets might be used. Kim et al. [96][61] employed datasets for decision rule discovery by creating decision trees after using PCA. They employed CART at this stage and applied these strategies to all clusters. According to clinical practice guidelines, they imposed the first split rules as the primary source of damage.4.2.4. K-Nearest Neighbor
The patient populations with the smallest sample sizes, which are those with significant cognitive deficits, show the lowest classifications. The classification is quite appropriate for the remaining groups, where a particular diagnosis is needed to make future healthcare plans. These results imply that the degree of cognitive impairment in PD patients can be assessed using EEG parameters derived from a daily clinical practice exploratory research approach [98][62].For each test sample, K-Nearest Neighbor (KNN) is a fundamental classification algorithm that produces the most comparable clusters among the K closest examples in the training set [99][63]. Bind et al. [16][6] developed a classification strategy based on KNN to predict voice signals to detect Parkinson’s sickness or healthy patients using a Parkinson’s speech dataset with various audio recordings. It had an accuracy of 80%. To handle the problem of categorizing Parkinson’s patients’ speech, data mining techniques, including Random Forest, Ada-Boost, and K-NN, were used by AH Al-F4.2.5. K-Mean Clustering
K-mean is a known approach for separating PD patients into subgroups, such as those with tremor predominance vs. those with fast motor control loss and cognitive issues. An overfitted system may have too many customizable variables, causing unpredictability or other confusion in training examples to be misinterpreted as true disease-related architecture. Because the model’s intricacy may be indefinitely raised to achieve high accuracy, this is a common challenge in statistical ML. With encouraging findings, some researchers have used dynamic handwriting analysis to categorize persons with PD. Regardless of the amount of sickness indicated by the patients, they all focused on the healthy/unhealthy binary distinction.4.2.6. Random Forest
Novel PD data with a class-balanced distribution were classified using the RF classification and the SMOTE method, modeling the data using the data points using multiple decision trees. New predictions were created by combining the findings of each decision tree and giving that category to the data point that was predicted by the majority of the trees [58][31]. Medication doses, time variables, and preoperative symptom-specific levodopa response were all shown to be strongly linked with clinical outcomes [90][64].4.2.7. Support Vector Machine
Ref. [48][24] included several statistical features collected from time-series gait data were examined before being decreased using a correlation matrix. The top seven feature vectors were then extracted and classified using a kernel-based SVM decoder and a Gaussian radial basis function. The findings showed that the seven features used for SVM had a precision of 83.33%, a high PD detection rate of 75%, and a low false positive rate of 16.67%. In an unlimited dimension space, an SVM creates a hyperplane that may be used for classification or regression. The classifier with the least errors is the one with the biggest gap between data points. It is used to classify the extracted characteristics. Leave-one-out cross-validation is used once more for training. Research has been conducted for resting tremors, but they also looked at postural and mixed tremor performance [102][65]. An algorithm was developed to categorize feature vectors that included probability, as well as other data, using other statistics.4.2.8. Ensemble Models
Ensemble models demonstrated that some of the features employed can detect indications of PD while being undetectable to human ears. This is a very hopeful discovery for the field, as it suggests that a large robust model could someday outperform humans. This also demonstrates the importance of voice phonation. Characteristics could be included in a set of non-invasive indicators for PD [103][66]. Dropout achieves the same result as the bagging ensemble strategy for a large number of DL models while maintaining low computing costs. The primary idea underlying dropout is that each time a new instance is input to the model, only a random subset of the network is used; consequently, the only parameter to tune is the likelihood of deleting a neuron [46][21]. Using structural MR images, ML was employed to differentiate PD from progressive supranuclear palsy (PSP), and a method based on resting-state brain networks was used to tell patients with Parkinson’s disease and those with very mild cognitive impairment apart.4.2.9. Limitations of the ML Models
Although earlier research has examined the application of machine learning in the diagnosis and evaluation of Parkinson’s disease (PD), studies have only been able to analyze data from wearable sensors, kinematics, and motor functions [30,104,105][14][67][68]. The lack of adequate or accurate descriptions of techniques or findings, as well as some research’s failure to accurately report the number and kind of subjects utilized or how ML models were implemented, trained, and assessed, were problems seen in many of the included research.4.2.10. Inference of ML Models
In summary, the realization of machine-learning-assisted diagnosis of PD yields high potential for a more systematic clinical decision-making system, while the adaptation of novel biomarkers may give rise to easier access to PD diagnosis at an earlier stage. Machine learning approaches, therefore, have the potential to provide clinicians with additional tools to screen, detect, or diagnose PD.4.3. Deep Learning Models
4.3.1. Recurrent Neural Networks
To enable the hard customized prediction task, Che et al. [111][69] presented an RNN design to calculate the commonalities connecting the health records segments with a DTW-similar architecture that brings superior alignment for periods with substantial temporal changes. According to these findings, the RNN representation with the ADAM algorithm produced the finest classification results on both voice sets. These outcomes demonstrate the advantages of LSTM and ADAM optimization together. The model was evaluated using a variety of criteria and was tested on two different speech datasets. The accuracy was 95.8%, retention was 100%, accuracy was 92.3%, and the F-score was 96% on the first dataset [46][21]. RNNs are used to process sequences for text mining because they keep track of past hidden layer processing memory. Because the training converges more quickly and recognizes long-term patterns in the data, LSTM is far superior to basic RNN units [112][70]. DL methods and word embedding models have been explored to interpret and analyze user perspectives on Parkinson’s illness [113][71]. Multiple nodes are found in the RNN’s hidden layer [114][72].4.3.2. Deep Autoencoder
An autoencoder is a program that can be used to learn representations, reduce complexity, and condense data [115][73]. In unlabeled data learning and voice identification applications, an autoencoder [116][74] has been widely used. The input data, dense nodes, and reconstructed surfaces can all be created as a three-layer neural system [53][26]. To learn about patient representations, deep learning techniques were used.4.3.3. Long Short-Term Memory
The rear-diffusion method is used by LSTM for training. In an LSTM network, there are three valves. The input, forget, and output gates are the three gates. To select whether input data should be activated and changed in the store, the input gate employs a logistic function. For both the DNN and LSTM studies, the performance indicators obtained for each of these systems were utilized to assess statistics quantifiers, such as average, mean, variance, and so on. The efficiency of the LSTM model was 99.03%, with a standard deviation of less than 1%, which means that most accuracy measurements were around 97.96%, which is significantly better than the results of the majority of previous research in this sector. The DNN algorithms showed a range of min and max levels of accuracy of 90 to 97%, which was better than earlier research, but not as good as the LSTM models. With the greatest accuracy of 97.12% and 99.03%, on the same dataset, the DNN and LSTM-based prediction models performed better than all other models, indicating that these are trustworthy models for detecting PD [83][75].4.3.4. Deep Neural Network
A DNN is composed of several basic components that are built on top of each other. Most of these simple structures perform irregular operations, like rescaling information to depict it in a different dimension, which helps uncover concealed features in the data [118,119,120][76][77][78]. The DNN proposed in this research is made up of two basic components that are coupled together: SAE, as well as a SoftMax predictor. SAE [121][79] is formed when the required number of autoencoders is combined.4.3.5. Deep Belief Network
DBNs (Deep Belief Networks) are a form of DNN that models high-level representation in the database with complicated composition using several computational levels [122][80]. These processing levels are linked by connection weights, but there are no connections between them. As a result, it is a generative graphical model [123][81] that is made up of numerous layers of hidden units. The evident surface is something that will collect the data (pattern characteristics) and will be altered at many processing levels. The quantity of packages in the viewable gradient increases to 16 neurons when 16 of the features collected by [124][82] are taken into account. Because the algorithm will categorize the waveform into each of the two risks, normal (0) or sick (1), only one system is necessary for the output layer. According to G. Hinton [123][81], the number of training examples, their complexity, and duplication might impact the number of these components. The DBN technique is a sort of NN approach. The layers are completely connected; however, there are no connections between the inner layers. These processing levels are linked together by connection weights, but there is no connection between them. As a result, it is a visual model made up of many surfaces of concealed neurons [125][83].4.3.6. Deep Convolutional Neural Network
Traditional PD detection approaches are typically handmade and need a high level of knowledge. The CNN uses an alternating convolution and pooling layer structure instead of completely linked hidden layers. They have been utilized in speech and audio processing for a variety of applications, including pathological speech categorization, audio activity recognition, voice identification, and more. CNNs are meant to handle datasets from multiple matrices, such as a three-channel color picture (RGB) or two-dimensional arrays that correlate to the TFR (Time-Frequency Response) of sound transmissions. The hidden projections of the neural network may be interpreted using the extracted features discovered by the CNN trained on multimodal input. The CNN’s initial convolutional layers, which were trained with speech TFRs, exhibit substantial disparities between PD sufferers and HC. The last layer of the CNN is trained using handwriting, producing similar results. It appears to be a good fit for modeling PD patients’ difficulty in starting and stopping distinct limb motions, allowing for the reliable categorization of PD patients and HC controls. Furthermore, the proposed designs appear to have the potential to classify various phases of the illness. Their suggested CNN directly and automatically creates feature representations using parallel convolution layers matching each feature set. This was the first research to use a CNN with comparable surfaces to identify PD. Scholars have proposed utilizing a wrist-mounted accelerometer and gyroscope to collect tremor data, with CNN networks used to classify the data. The approach was tested on 92 patients and was found to be 85% accurate. The learned features are obtained by convolving the input data with a variety of filters during the training process [102,128,129][65][84][85]. They used a CNN to analyze spiral and meandering hand sketching characteristics in PD patients and found that the accuracy for 128 × 128 meander pictures was 87.14% and 77.92% for 128 × 128 spiral images [64][35]. Ref. [130][86] suggested a data augmentation method using a combination of GANs and Alex-Net that will successfully produce high-quality MR images and increase the performance of the classification model. A useful reference is provided by this work in medical image evaluation using DL. Srivastava [4] created a hybrid CNN-LSTM model in which the CNN learns well from spatial features from stride data and the LSTM trains well from the sufferer’s time factors to forecast the intensity of the condition. When compared to base models for classification, this spatiotemporal model provided better results. By calculating an anemic person’s blood count test results using optimal CNN and SAE with GA, they divided anemia patients into three groups. It demonstrated that their model was 98% more accurate than baseline models [131][87]. DCNN has considerably increased picture categorization and detection performance. Deep learning algorithms for segmentation, tumor identification, and disease classification have recently been applied to medical pictures. Scientists have used TFR and CNN to describe PD patients’ articulation deficits. J.C. Vasquez-Correa et al. [132][88] identified PD and HC participants using voice recordings in three languages: Spanish, German, and Czech, with a degree of precision ranging from 70% to 85% based on the language, suggesting that learning techniques have the potential for analyzing the speech of patients.4.3.7. Deep Generative Models
Deep Generative Model (DGM) designs are challenging to implement due to their tremendous mathematical complexity. Several organizations have been working on systems that incorporate the core architectures of deep learning, making learning, configuration, and other uses of these tools in massive amounts of data easier [134][89]. Addressing these obstacles through analytical means would have a substantial impact on the treatment of PD and other neurodegenerative disorders, where evaluation faces similar issues. In ubiquitous computing, their system includes a standard analytic pipeline for activity recognition. The collected data is first segmented using a sliding window process, followed by the extraction of a hand-crafted collection of characteristics from each frame.4.3.8. Deep Boltzmann Machine
Passos et al. [135][90] addressed the topic of a fine-tuning DBM to reconstruct binary pictures using meta-heuristic-driven optimization strategies. When compared to a random search, the experimental findings from three public databases demonstrated the validity of applying such strategies to optimize DBMS. They also demonstrated that when two out of three datasets were used, DBMs might develop better accurate estimates than DBNs. Wilcoxon signed-rank analysis was also employed to look at the similarities between each optimization approach, as well as the exchange between the computational burden imposed by each heuristic algorithm and its efficacy. The Continuous RBM successfully learns a deep cortical signal representation. For some patients, the CRBM model detects HVS (High-Voltage Spindles) before the ground truth; the lower the specificity, the sooner the HVS is detected. Because of the signal-to-noise ratio per channel or the existence of HVS, data quality can greatly vary from one rat to the next. The continuous RBM’s properties offer a variety of benefits, and there are currently several ways to improve it. It is an unsupervised generative model that may be used as a predictor and can learn ideal frequencies to detect. It can extract quasi-components, but the data must be separable. To assist hidden units in extracting different components, a knowledge of the model’s architecture is required.4.3.9. Deep Reinforcement Learning
Reinforcement Learning (RL) discipline optimizes subsequent choice tasks based on predetermined outcomes. It is one of the three branches of machine learning (along with supervised and unsupervised training). Researchers have discovered that the RL method generates a pharmaceutical schedule that is equivalent to that of physicians. They used a multivariate regression model to establish that health assessment ratings and medications had a significant enough level in forecasting future UPDRS III. Then, utilizing the statistically relevant factors and decision tree regressor, they created 28 distinct illness conditions that correlated to another overall UPDRS III score. RL can help PD patients improve their pharmaceutical strategy by suggesting treatments that are both efficient and effective. When general neurologists and primary care physicians deal with difficult cases where the appropriate drug combination is in dispute, this model will be tremendously useful. This effort marks the start of the creation of an AI-physician ecosystem that is collaborative [128][84]. They use deep RL to resolve the resultant model (DRL). The ideal treatment strategy that minimizes the patient’s symptoms is determined by the recommended policy. Their findings reveal that the prototype strategy beats the static a priori therapy plan as part of alleviating patient symptoms, demonstrating that DRL may be used to supplement medical decision-making for chronic illness treatment decisions. A3C is a DRL method that employs an actor to interact with the surroundings and a critic to learn about function and policy. The A3C approach makes use of numerous concurrent agent threads engaging with environment replicas, each of which asynchronously updates a world net [139][91]. In medicine, they have demonstrated stronger learning from positive reinforcement and poorer learning from negative reinforcement, whereas individuals who were not on medication showed the reverse trend. Patients who were not on medication performed much better at negative reinforcement than normal age controls (HC), implying that PD enhanced some components of RL. Dopamine may alter learning expression, according to a recent update to typical RL models. The active and passive routes, which learn from positive and negative reinforcement, have independent learning rates and factors that can influence the OpAL model. Enabling dopamine to influence the decision parameter can lead to a bias toward picking stimuli primarily learned through the direct or indirect pathways, giving greater weight to the rewards or punishments acquired.4.3.10. Extreme Learning Machine
An ELM has a very quick learning rate. The input weights are chosen at random using SLFNs, and the output weight is analytically calculated. Not only are the hidden node parameters free of the training examples, but they are also independent of one another. It may be possible to generate the concealed node without considering the training data. All nonlinear piece—wise constant functions are compatible with ELM. PD has been predicted using the newly developed Meta-cognitive Fully Complex-valued Radial Basis Function (McFCRBF) network. When contrasted with a real-valued ELM and the FC-RBF network, the effectiveness of the Mc-FCRBF used to predict PD demonstrates that it predicts the illness better. The meta-cognitive product’s self-regulatory learning process is credited with enhanced quality [141][92]. Nagasubramanian and Sankayya [71][51] tested ELM algorithms for PD categorization. The sigmoid function was used in the extreme training algorithm, and it was quick to operate. In an ELM system, the real-valued inputs and objectives were applied to the network. Regarding precision, the Mc-FCRBF network performed better than the ELM and FC-RBF networks. Consequently, BCGA-ELM could effectively distinguish between troublesome and ideal solutions. The results of the experiments clearly showed that the suggested technique could provide a similar solution for the PD classification issue for various random initializations. They intend to conduct a medical investigation of the 19 genes they chose in their upcoming research [78][53].4.3.11. Limitations of the DL Models
To assist physicians in their choices, this in-depth analysis highlights the information on diagnosing Parkinson’s disease (PD). It is acknowledged that gathering real-world data from patients is the most difficult endeavor in the healthcare sector compared to other study sectors. The medical datasets collected for any neurodegenerative condition are typically unbalanced.-
Given that the imbalanced dataset today influences the results, handling it is quite difficult.
-
In addition, due to advancements in deep learning techniques combined with nature-inspired methodologies, there is a latent potential to leverage multimodal datasets to enhance PD’s prediction accuracy.
-
Although using the right criteria to assess ML models’ performance in PD classification is important, there is still room for improvement.
4.3.12. Inferences of DL Models
A variety of studies have been conducted to determine the viability of various machine learning methods. Cross-validation methods were used to choose the most crucially dependable models. Most of the designs followed non-motor features, but some models employed motor aspects that were more enhancing than others. Using these kinds of models to detect diseases has many advantages. In other cases, PD was detected by analyzing the affected people’s handwriting. The other means of identifying the same is through missing data. Therefore, each of these approaches deals with a separate set of conclusions and seeks to provide a thorough examination of this specific subject in a variety of ways.References
- Parkinson Association of the Carolinas. Understanding Parkinson’s Disease—Parkinson’s Association of Carolinas. 2022. Available online: https://www.parkinsonassociation.org/understanding-parkinsons-disease/ (accessed on 20 December 2022).
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386.
- Frid, A.; Hazan, H.; Hilu, D.; Manevitz, L.; Ramig, L.O.; Sapir, S. Computational Diagnosis of Parkinson’s Disease Directly from Natural Speech Using Machine Learning Techniques. In Proceedings of the 2014 IEEE International Conference on Software Science, Technology and Engineering, Ramat Gan, Israel, 11–12 June 2014; pp. 50–53.
- Srivastava, S. Genetic Algorithm Optimized Deep Learning Model for Parkinson Disease Severity Detection. Ph.D. Thesis, National College of Ireland, Dublin, Ireland, 2021.
- Ahmadi Rastegar, D.; Ho, N.; Halliday, G.M.; Dzamko, N. Parkinson’s progression prediction using machine learning and serum cytokines. NPJ Park. Dis. 2019, 5, 14.
- Bind, S.; Tiwari, A.K.; Sahani, A.K. A survey of machine learning based approaches for Parkinson disease prediction. Int. J. Comput. Sci. Inf. Technol. 2015, 6, 1648–1655.
- Armstrong, M.J.; Okun, M.S. Time for a new image of Parkinson disease. JAMA Neurol. 2020, 77, 1345–1346.
- Cho, C.W.; Chao, W.H.; Lin, S.H.; Chen, Y.Y. A vision-based analysis system for gait recognition in patients with Parkinson’s disease. Expert Syst. Appl. 2009, 36, 7033–7039.
- Johri, A.; Tripathi, A. Parkinson Disease Detection Using Deep Neural Networks. In Proceedings of the 2019 Twelfth International Conference on Contemporary Computing (IC3), Noida, India, 8–10 August 2019; pp. 1–4.
- El Maachi, I.; Bilodeau, G.A.; Bouachir, W. Deep 1D-Convnet for accurate Parkinson disease detection and severity prediction from gait. Expert Syst. Appl. 2020, 143, 113075.
- Camps, J.; Sama, A.; Martin, M.; Rodriguez-Martin, D.; Perez-Lopez, C.; Arostegui, J.M.M.; Cabestany, J.; Català, A.; Alcaine, S.; Mestre, B.; et al. Deep learning for FOG detection in Parkinson’s disease patients in their homes using a waist-worn inertial measurement unit. Knowl.-Based Syst. 2018, 139, 119–131.
- Thomas, M.; Lenka, A.; Kumar Pal, P. Handwriting analysis in Parkinson’s disease: Current status and future directions. Mov. Disord. Clin. Pract. 2017, 4, 806–818.
- Kubota, K.J.; Chen, J.A.; Little, M.A. Machine learning for large-scale wearable sensor data in Parkinson’s disease: Concepts, promises, pitfalls, and futures. Mov. Disord. 2016, 31, 1314–1326.
- Ahlrichs, C.; Lawo, M. Parkinson’s disease motor symptoms in machine learning: A review. arXiv 2013, arXiv:1312.3825.
- Sherrill, D.M.; Hughes, R.; Salles, S.S.; Lie-Nemeth, T.; Akay, M.; Standaert, D.G.; Bonato, P. Advanced Analysis of Wearable Sensor Data to Adjust Medication Intake in Patients with Parkinson’s Disease. In Proceedings of the 2005 Neural Engineering—Conference Proceedings: 2nd International IEEE EMBS Conference, Arlington, VA, USA, 16–19 March 2005; pp. 202–205.
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J.Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. Available online: http://jnnp.bmj.com/content/79/4/368.abstract (accessed on 18 December 2022).
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170.
- Cook, D.J.; Schmitter-Edgecombe, M.; Dawadi, P. Analyzing activity behavior and movement in a naturalistic environment using smart home techniques. IEEE J. Biomed. Health Inform. 2015, 19, 1882–1892.
- Bloem, B.R.; Hausdor, J.M.; Visser, J.E.; Giladi, N. Falls and FOG in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. J. 2004, 19, 871–884.
- Giladi, N.; Tal, J.; Azulay, T.; Rascol, O.; Brooks, D.J.; Melamed, E.; Oertel, W.; Poewe, W.H.; Stocchi, F.; Tolosa, E. Validation of the FOG questionnaire in patients with Parkinson’s disease. Mov. Disord. J. 2009, 24, 655–661.
- Abd El Aal, H.A.; Taie, S.A.; El-Bendary, N. An optimized RNN-LSTM approach for Parkinson’s disease early detection using speech features. Bull. Electr. Eng. Inform. 2021, 10, 2503–2512.
- Senthilarumugam Veilukandammal, M.; Nilakanta, S.; Ganapathysubramanian, B.; Anantharam, V.; Kanthasamy, A.; Willette, A. Big Data and Parkinson’s Disease: Exploration, Analyses, and Data Challenges. In Proceedings of the 51st Hawaii International Conference on System Sciences, Hilton Waikoloa Village, HI, USA, 3–6 January 2018.
- Bazgir, O.; Frounchi, J.; Habibi SA, H.; Palma, L.; Pierleoni, P. A Neural Network System for Diagnosis and Assessment of Tremor in Parkinson Disease Patients. In Proceedings of the 2015 22nd Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 25–27 November 2015; pp. 1–5.
- Shetty, S.; Rao, Y.S. SVM Based Machine Learning Approach to Identify Parkinson’s Disease Using Gait Analysis. In Proceedings of the 2016 International Conference on Inventive Computation Technologies (ICICT), Coimbatore, India, 26–27 August 2016; Volume 2, pp. 1–5.
- Oung, Q.W.; Hariharan, M.; Lee, H.L.; Basah, S.N.; Sarillee, M.; Lee, C.H. Wearable Multimodal Sensors for Evaluation of Patients with Parkinson Disease. In Proceedings of the 2015 IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 27–29 November 2015; pp. 269–274.
- Zhang, Y.N. Can a smartphone diagnose Parkinson disease? A deep neural network method and telediagnosis system implementation. Park. Dis. 2017, 2017, 6209703.
- Abayomi-Alli, O.O.; Damaševičius, R.; Maskeliūnas, R.; Abayomi-Alli, A. BiLSTM with Data Augmentation Using Inter Ion Methods to Improve Early Detection of Parkinson Disease. In Proceedings of the 2020 15th Conference on Computer Science and Information Systems (FedCSIS), Sofia, Bulgaria, 6–9 September 2020; pp. 371–380.
- Al-Fatlawi, A.H.; Jabardi, M.H.; Ling, S.H. Efficient Diagnosis System for Parkinson’s Disease Using Deep Belief Network. In Proceedings of the 2016 IEEE Congress on Evolutionary Computation (CEC), Vancouver, BC, Canada, 24–29 July 2016; pp. 1324–1330.
- Hazan, H.; Hilu, D.; Manevitz, L.; Ramig, L.O.; Sapir, S. Early Diagnosis of Parkinson’s Disease via Machine Learning on Speech Data. In Proceedings of the 2012 IEEE 27th Convention of Electrical and Electronics Engineers in Israel, Eilat, Israel, 14–17 November 2012; pp. 1–4.
- McNamara, P.; Obler, L.K.; Au, R.; Durso, R.; Albert, M.L. Speech monitoring skills in Alzheimer’s disease, Parkinson’s disease, and normal aging. Brain Lang. 1992, 42, 38–51.
- Karan, B.; Sahu, S.S.; Mahto, K. Parkinson disease prediction using intrinsic mode function-based features from speech signal. Biocybern. Biomed. Eng. 2020, 40, 249–264.
- Caliskan, A.; Badem, H.; Basturk, A.; Yuksel, M.E. Diagnosis of the Parkinson disease by using deep neural network classifier. IU-J. Electr. Electron. Eng. 2017, 17, 3311–3318.
- Mandal, I.; Sairam, N. New machine-learning algorithms for prediction of Parkinson’s disease. Int. J. Syst. Sci. 2014, 45, 647–666.
- Kotsenas, A.L.; Vernooij, M.W.; Port, J.D. Advances in neurodegenerative and psychiatric imaging: Introductory editorial. Br. J. Radiol. 2019, 92, 20199003.
- Pereira, C.R.; Weber, S.A.; Hook, C.; Rosa, G.H.; Papa, J.P. Deep Learning-Aided Parkinson’s Disease Diagnosis from Handwritten Dynamics. In Proceedings of the 2016 29th SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Sao Paulo, Brazil, 4–7 October 2016; pp. 340–346.
- Jin, B.; Qu, Y.; Zhang, L.; Gao, Z. Diagnosing Parkinson disease through facial expression recognition: Video analysis. J. Med. Internet Res. 2020, 22, e18697.
- Nieuwboer, A.; Dom, R.; De Weerdt, W.; Desloovere, K.; Janssens, L.; Stijn, V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain 2004, 127, 1650–1660.
- Misiaszek, G.; Riconscente, M.; Henke, M.; Walsh, J.P. Online multimedia teaching tool for Parkinson’s disease. J. Undergrad. Neurosci. Educ. 2008, 6, A68.
- Faulkner, T.P.; Sprague, J.E. Application of several multimedia approaches to the teaching of CNS pharmacology: Parkinson’s disease and antiparkinsonism drugs. Am. J. Pharm. Educ. 1996, 60, 417–421.
- Yu, W.; Vuong, C.; Ingalls, T. An Interactive Multimedia System for Parkinson’s Patient Rehabilitation. In International Conference on Virtual and Mixed Reality; Springer: Berlin/Heidelberg, Germany, 2020; pp. 129–137.
- Shen, T.; Jiang, J.; Lin, W.; Ge, J.; Wu, P.; Zhou, Y.; Zuo, C.; Wang, J.; Yan, Z.; Shi, K. Use of overlapping group LASSO sparse deep belief network to discriminate Parkinson’s disease and normal control. Front. Neurosci. 2019, 13, 396.
- Parkinson’s Foundation. Stages of Parkinson’s. 2023. Available online: https://www.parkinson.org/understanding-parkinsons/what-is-parkinsons/stages (accessed on 31 January 2023).
- 5 Stages of Parkinson’s Disease. 2023. Available online: https://www.healthline.com/health/parkinsons/stages (accessed on 31 January 2023).
- Drotár, P.; Mekyska, J.; Rektorová, I.; Masarová, L.; Smékal, Z.; Faundez-Zanuy, M. Evaluation of handwriting kinematics and pressure for differential diagnosis of Parkinson’s disease. Artif. Intell. Med. 2016, 67, 39–46.
- Pereira, C.R.; Pereira, D.R.; Weber, S.A.; Hook, C.; De Albuquerque VH, C.; Papa, J.P. A survey on computer-assisted Parkinson’s disease diagnosis. Artif. Intell. Med. 2019, 95, 48–63.
- Geerse, D.J.; Roerdink, M.; Marinus, J.; Van Hilten, J.J. Assessing Walking Adaptability in Parkinson’s Disease: The Interactive Walkway. Front. Neurol. 2018, 9, 1096.
- Loh, H.W.; Hong, W.; Ooi, C.P.; Chakraborty, S.; Barua, P.D.; Deo, R.C.; Soar, J.; Palmer, E.E.; Acharya, U.R. Application of deep learning models for automated identification of Parkinson’s disease: A review (2011–2021). Sensors 2021, 21, 7034.
- Heinzel, S.; Roeben, B.; Ben-Shlomo, Y.; Lerche, S.; Alves, G.; Barone, P.; Behnke, S.; Berendse, H.W.; Bloem, B.R.; Burn, D.; et al. Prodromal markers in Parkinson’s disease: Limitations in longitudinal studies and lessons learned. Front. Aging Neurosci. 2016, 8, 147.
- Butt, A.H.; Rovini, E.; Dolciotti, C.; De Petris, G.; Bongioanni, P.; Carboncini, M.C.; Cavallo, F. Objective and automatic classification of Parkinson disease with Leap Motion controller. Biomed. Eng. Online 2018, 17, 168.
- Tiwari, H.; Shridhar, S.K.; Patil, P.V.; Sinchana, K.R.; Aishwarya, G. Early prediction of Parkinson disease using machine learning and deep learning approaches. EasyChair Prepr. 2021, 4889, 1–14.
- Nagasubramanian, G.; Sankayya, M. Multi-variate vocal data analysis for detection of Parkinson disease using deep learning. Neural Comput. Appl. 2021, 33, 4849–4864.
- Aich, S.; Kim, H.C.; Hui, K.L.; Al-Absi, A.A.; Sain, M. A Supervised Machine Learning Approach Using Different Feature Selection Techniques on Voice Datasets for Prediction of Parkinson’s Disease. In Proceedings of the 2019 21st International Conference on Advanced Communication Technology (ICACT), PyeongChang, Republic of Korea, 17–20 February 2019; pp. 1116–1121.
- Sachnev, V.; Kim, H.J. Parkinson Disease Classification Based on Binary Coded Genetic Algorithm and Extreme Learning Machine. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 21–24 April 2014; pp. 1–6.
- García, A.M.; Arias-Vergara, T.; CVasquez-Correa, J.; Nöth, E.; Schuster, M.; Welch, A.E.; Bocanegra, Y.; Baena, A.; Orozco-Arroyave, J.R. Cognitive determinants of dysarthria in Parkinson’s disease: An automated machine learning approach. Mov. Disord. 2021, 36, 2862–2873.
- Tiwari, A.K. Machine learning based approaches for prediction of Parkinson’s disease. Mach. Learn. Appl. 2016, 3, 33–39.
- Darnall, N.D.; Donovan, C.K.; Aktar, S.; Tseng, H.Y.; Barthelmess, P.; Cohen, P.R.; Lin, D.C. Application of machine learning and numerical analysis to classify tremor in patients affected with essential tremor or Parkinson’s disease. Gerontechnology 2012, 10, 208–219.
- Zham, P.; Arjunan, S.P.; Raghav, S.; Kumar, D.K. Efficacy of guided spiral drawing in the classification of Parkinson’s disease. IEEE J. Biomed. Health Inform. 2017, 22, 1648–1652.
- Marar, S.; Swain, D.; Hiwarkar, V.; Motwani, N.; Awari, A. Predicting the Occurrence of Parkinson’s Disease Using Various Classification Models. In Proceedings of the 2018 International Conference on Advanced Computation and Telecommunication (ICACAT), Bhopal, India, 28–29 December 2018; pp. 1–5.
- Miljkovic, D.; Aleksovski, D.; Podpečan, V.; Lavrač, N.; Malle, B.; Holzinger, A. Machine Learning and Data Mining Methods for Managing Parkinson’s Disease. In Machine Learning for Health Informatics; Springer: Cham, Switzerland, 2016; pp. 209–220.
- Nilashi, M.; bin Ibrahim, O.; Ahmadi, H.; Shahmoradi, L. An analytical method for diseases prediction using machine learning techniques. Comput. Chem. Eng. 2017, 106, 212–223.
- Kim, Y.; Suescun, J.; Schiess, M.C.; Jiang, X. Computational medication regimen for Parkinson’s disease using reinforcement learning. Sci. Rep. 2021, 11, 9313.
- Betrouni, N.; Delval, A.; Chaton, L.; Defebvre, L.; Duits, A.; Moonen, A.; Leentjens, A.F.G.; Dujardin, K. Electroencephalography-based machine learning for cognitive profiling in Parkinson’s disease: Preliminary results. Mov. Disord. 2019, 34, 210–217.
- Impedovo, D.; Pirlo, G.; Vessio, G. Dynamic handwriting analysis for supporting earlier Parkinson’s disease diagnosis. Information 2018, 9, 247.
- Shamir, R.R.; Dolber, T.; Noecker, A.M.; Walter, B.L.; McIntyre, C.C. Machine learning approach to optimizing combined stimulation and medication therapies for Parkinson’s disease. Brain Stimul. 2015, 8, 1025–1032.
- Oktay, A.B.; Kocer, A. Differential diagnosis of Parkinson and essential tremor with convolutional LSTM networks. Biomed. Signal Process. Control. 2020, 56, 101683.
- Wang, M.; Ge, W.; Apthorp, D.; Suominen, H. Robust feature engineering for Parkinson disease diagnosis: New machine learning techniques. JMIR Biomed. Eng. 2020, 5, e13611.
- Ramdhani, R.A.; Khojandi, A.; Shylo, O.; Kopell, B.H. Optimizing clinical assessments in Parkinson’s disease through the use of wearable sensors and data driven modeling. Front. Comput. Neurosci. 2018, 12, 72.
- Belić, M.; Bobić, V.; Badža, M.; Šolaja, N.; Đurić-Jovičić, M.; Kostić, V. Artificial intelligence for assisting diagnostics and assessment of Parkinson’s disease—A review. Clin. Neurol. Neurosurg. 2019, 184, 105442.
- Che, C.; Xiao, C.; Liang, J.; Jin, B.; Zho, J.; Wang, F. An RNN Architecture with Dynamic Temporal Matching for Personalized Predictions of Parkinson’s Disease. In Proceedings of the 2017 SIAM International Conference on Data Mining, Houston, TX, USA, 27–29 April 2017; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA; pp. 198–206.
- Kuresan, H.; Samiappan, D.; Jeevan, A.; Gupta, S. A Performance Study of ML Models and Neural Networks for Detection of Parkinson Disease using Dysarthria Symptoms. Eur. J. Mol. Clin. Med. 2021, 8, 2021.
- Afonso, L.C.; Rosa, G.H.; Pereira, C.R.; Weber, S.A.; Hook, C.; Albuquerque VH, C.; Papa, J.P. A recurrence plot-based approach for Parkinson’s disease identification. Future Gener. Comput. Syst. 2019, 94, 282–292.
- Haller, S.; Badoud, S.; Nguyen, D.; Garibotto, V.; Lovblad, K.O.; Burkhard, P.R. Individual detection of patients with Parkinson disease using support vector machine analysis of diffusion tensor imaging data: Initial results. Am. J. Neuroradiol. 2012, 33, 2123–2128.
- Prince, J.; De Vos, M. A Deep Learning Framework for the Remote Detection of Parkinson’s Disease Using Smart-Phone Sensor Data. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3144–3147.
- Hoehn, M.; Yahr, M. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427.
- Rizvi, D.R.; Nissar, I.; Masood, S.; Ahmed, M.; Ahmad, F. An LSTM based Deep learning model for voice-based detection of Parkinson’s disease. Int. J. Adv. Sci. Technol. 2020, 29, 337–343.
- Swarna, S.R.; Kumar, A.; Dixit, P.; Sairam, T.V.M. Parkinson’s Disease Prediction Using Adaptive Quantum Computing. In Proceedings of the 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, 4–6 February 2021; pp. 1396–1401.
- Bengio, Y. Practical Recommendations for Gradient-Based Training of Deep Architectures. Neural Networks: Tricks of the Trade; Springer: Berlin/Heidelberg, Germany, 2012; pp. 437–478.
- Le, Q.; Ngiam, J.; Coates, A.; Lahiri, A.; Prochnow, B.; Ng, A. On Optimization Methods for Deep Learning. In Proceedings of the 28th International Conference on Machine Learning (ICML-11), Bellevue, DC, USA, 28 June–2 July 2011; pp. 265–272.
- Severson, K.A.; Chahine, L.M.; Smolensky, L.A.; Dhuliawala, M.; Frasier, M.; Ng, K.; Ghosh, S.; Hu, J. Discovery of Parkinson’s disease states and disease progression modelling: A longitudinal data study using machine learning. Lancet Digit. Health 2021, 3, e555–e564.
- Deng, L.; Yu, D. Deep learning: Methods and applications. Found. Trends Signal Process. 2014, 7, 197–387.
- Hinton, G. A practical guide to training restricted Boltzmann machines. Momentum 2010, 9, 926.
- Little, M.A.; McSharry, P.E.; Hunter, E.J.; Spielman, J.; Ramig, L. Suitability of Dysphonia Measurements for Telemonitoring of Parkinson Disease. IEEE Trans. Biomed. Eng. 2008, 56, 1015–1022.
- Nilashi, M.; Ahmadi, H.; Sheikhtaheri, A.; Naemi, R.; Alotaibi, R.; Alarood, A.A.; Munshi, A.; Rashid, T.; Zhao, J. Remote tracking of Parkinson’s disease progression using ensembles of deep belief network and self-organizing map. Expert Syst. Appl. 2020, 159, 113562.
- Kim, H.B.; Lee, W.W.; Kim, A.; Lee, H.J.; Park, H.Y.; Jeon, H.S.; Kim, S.K.; Jeon, B.; Park, K.S. Wrist sensor-based tremor severity quantification in Parkinson’s disease using convolutional neural network. Comput. Biol. Med. 2018, 95, 140–146.
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016.
- Kaur, S.; Aggarwal, H.; Rani, R. Diagnosis of Parkinson’s disease using deep CNN with transfer learning and data augmentation. Multimed. Tools Appl. 2021, 80, 10113–10139.
- Kilicarslan, S.; Celik, M.; Sahin, S. Hybrid models based on genetic algorithm and deep learning algorithms for nutritional anemia disease classification. Biomed. Signal Process. Control 2021, 63, 102231.
- Vásquez-Correa, J.; Orozco-Arroyave, J.R.; Nöth, E. Convolutional Neural Network to Model Articulation Impairments in Patients with Parkinson’s Disease. In Proceedings of the INTERSPEECH, Stockholm, Sweden, 20–24 August 2017; pp. 314–318.
- Folador, J.P.; Andrade, A.O. Deep Learning Framework Used in Parkinson’s Disease Analysis. In Proceedings of the XI Simpósio de Engenharia Biomédica, Minas Gerais, Brazil, 20–24 August 2018.
- Passos, L.A.; Papa, J.P. A metaheuristic-driven approach to fine-tune deep Boltzmann machines. Appl. Soft Comput. 2020, 97, 105717.
- Watts, J.; Khojandi, A.; Vasudevan, R.; Ramdhani, R. Optimizing Individualized Treatment Planning for Parkinson’s Disease Using Deep Reinforcement Learning. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 20–24 July 2020; pp. 5406–5409.
- Gokul, S.; Sivachitra, M.; Vijayachitra, S. Parkinson’s Disease Prediction Using Machine Learning Approaches. In Proceedings of the 2013 Fifth International Conference on Advanced Computing (ICoAC), Chennai, India, 18–20 December 2013; pp. 246–252.
More
