Self-Selective Separation of Single-Walled Carbon Nanotubes via a Hydroxyl Group Reaction
We developed a method of separating metallic single-walled carbon nanotubes (SWNTs) from semiconducting nanotubes (NTs) in suspension using a sodium hydroxide treatment and centrifugation. The semiconducting NTs remained suspended in the solvent while the metallic NTs precipitated out. This self-selective separation occurred because the metallic SWNTs bonded to hydroxyl groups due to strong surface hydrogen-bond interactions. We fabricated thin-film transistors based on random networks of semiconducting SWNTs on a glass substrate and obtained an on/off current ratio of 6.25 x 104.
- Carbon nanotubes
- Nanotube devices
- Precipitation
- Semiconductor nanotubes
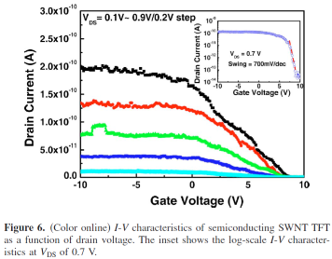
Figure 6 shows the current–voltage I-V curve characteristics of the SWNT TFT as a function of drain–source voltage VDS to illustrate the electrical characteristics of the semiconducting SWNT TFT in detail. The gate voltage VG varied over the 10 V range. The SWNT TFT behaved normally as a p-channel transistor. The on/off current ratio increased rapidly as VDS increased. The inset shows that the maximum transconductance gm = dID/dVG was about 6.4 x 10-11 A/V, and the on/off current ratio was approximately 6.25 x 104. The subthreshold slope {S = dVG / [d(log10 ID)]} was about 700 mV/decade at a VDS of 0.7 V. Thus, this method could be very useful for random networks of SWNT electronics.

