The gram-scale synthesis of Cu-poor CZTS nanocrystals (NCs) with tuned shape and composition, by using a facile phosphine-free heat-up procedure. Based on the detailed characterization and analyses on the evolution of crystal phases, composition, morphologies and optical spectra over the reaction stage and temperature, we explore the reaction mechanism. The synthesis reaction involves the formation of binary CuS (covellite) nanoparticles at room temperature, and the conversion from the as-formed CuS to intermediated Zn-Sn-codoped CuS NCs at 150 ºC and finally to quaternary CZTS NCs in kesterite phase at 220 ºC, during which the NCs gradually grow due to the progressive incorporation of foreign cations (Zn2+ and Sn2+) to the as-formed CuS NCs. The introduction of dodecanethiol (DT) leads to the formation of bullet-like CZTS NCs in wurtzite phase. The Cu-poor CZTS synthesis is reproducible by up-scaling the amounts of chemicals and solvent/ligand, which allows us for a promising approach of gram-scale production of Cu-based chalcohenides for potential applications in photovoltaics.
- Heat-up
- Gram-Scale
- CZTS Nanocrystals
Synthesis of pseudospherical and rice-like Cu-poor CZTS NCs
We noticed that the final fraction of each element in the resulting CZTS NCs didn’t match the molar ratios designed in the precursor. The Cu is rich and content of Zn is very low, which is due to the low reactivity of Zn2+ ions (relative to Cu and Sn) with S species.[1] Indeed, in a designed synthesis with stoichiometric precursor, that is, by fixing starting Cu:Zn:Sn:S molar ratio as 2:1:1:4, we observed that the atomic percentages of the final CZTS NCs is 27.6, 4.0, 15.8 and 52.6% (found by ICP analysis), respectively, indicating a composition of Cu2.1Zn0.3Sn1.2S4. Consequently, we increased the starting Zn2+ fraction and decreased the Cu2+ amount in the precursors in order to obtain Cu-poor CZTS NCs. The TEM/HRTEM images, XRD patterns and optical spectra of the representative samples are displayed in Fig. 1. As depicted in Fig. 1a-c, all of the resulting CZTS NCs are uniform in size. The majority of NPs were in pseudospherical shape when the precursor Cu:(Zn+Sn) ratio was in the range from 1:1.3 to 0.8:1.3 (Fig. 1a,b). However, some NPs were rice-like in case of starting Cu:(Zn+Sn) ratio below 0.6:1.3 (Fig. 1c). The analyses of XRD patterns ambiguously identified that kesterite hexagonal phase (JCPDS: 00-026-0575) of CZTS was formed both in the Cu-rich CZTS NCs and in the Cu-poor ones in case of starting Cu:(Zn+Sn) ratio higher than 0.6:1.3, without other phases or side-products formed in the resulting sample (Fig. 1g). The XRD patterns with peaks at 28.5°, 47.3°, and 56.1° could be indexed with the planes (112) (220) and (312) in kesterite phase of CZTS NCs. The HRTEM analyses (Fig. 1d-f) on the typical single NPs of the above three samples are in good accordance with the XRD identification. However, as the Cu-poor increased, secondary phase of orthorhombic SnS (JCPDS: 00-014-0620) was also identified from XRD analysis besides kesterite phase of CZTS (see the details in full paper CrystEngComm, 2017,19, 2013-2020), which indicated that the direct reaction between Sn2+ with S2- is not ignored in the presence of much more excess Sn precursor. We also noticed that in this case the resulting sample contained some pyramid-like NCs, which were further identified as orthorhombic phase of SnS NCs by HRTEM analysis.
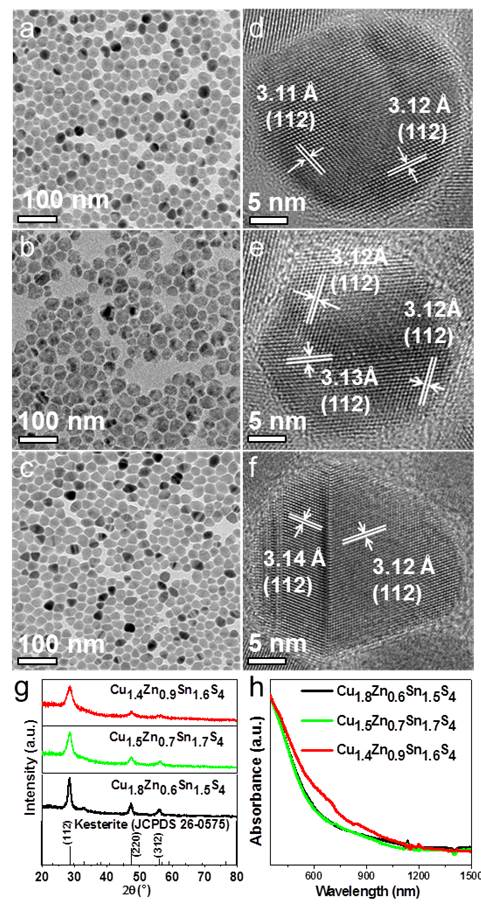
Fig. 1 Morphological (g) XRD patterns and (h) absorption spectra characterization of the representative Cu-poor CZTS samples and corresponding single nanocrystals.
Synthesis of Cu-poor bullet-like CZTS NCs
The reactivity of cation or anion precursors, reaction temperature and the ligand species were found to be important factors for the control of the crystallographic phase in synthesis of CZTS NCs.[1][2][3] It is interesting that by introduction of DT, we obtained bullet-like CZTS NCs, which evolved to wurtzite phase as DT amount increased (Fig. 2b-c). We noticed that the diffraction peaks at 28.5°, 47.3°, and 56.1° could be assigned to either the planes (112) (220) and (312) of kesterite or the planes (002) (110) and (112) of wurtzite phase, respectively. However, the emerging peaks at around 27.1°, 30.7° and 51.7° as DT increased to more than 2 mL are ambiguously indexed to the (100), (101) and (103) planes of wurtzite, respectively (Fig. 2g, green and red curves).[4][5] The attribution of wurtzite phase is further evidenced by HRTEM analyses on single NCs (Fig. 2e-f). The crystal planes in some single NCs are not regular due to the defects. The phase evolution in our work is in agreement with what observed by Li et al.,[2] that is, the highly reactive small molecule H2S generated by reaction of elemental sulfur with OM yielded the formation of kesterite phase, however, the reaction in the presence of long alkane chain DT led to the formation of wurtzite phase. Furthermore, the DT functioned as not only S precursor but also capping ligand, which tailored the growth of nuclei and led to the formation of bullet-like NPs.
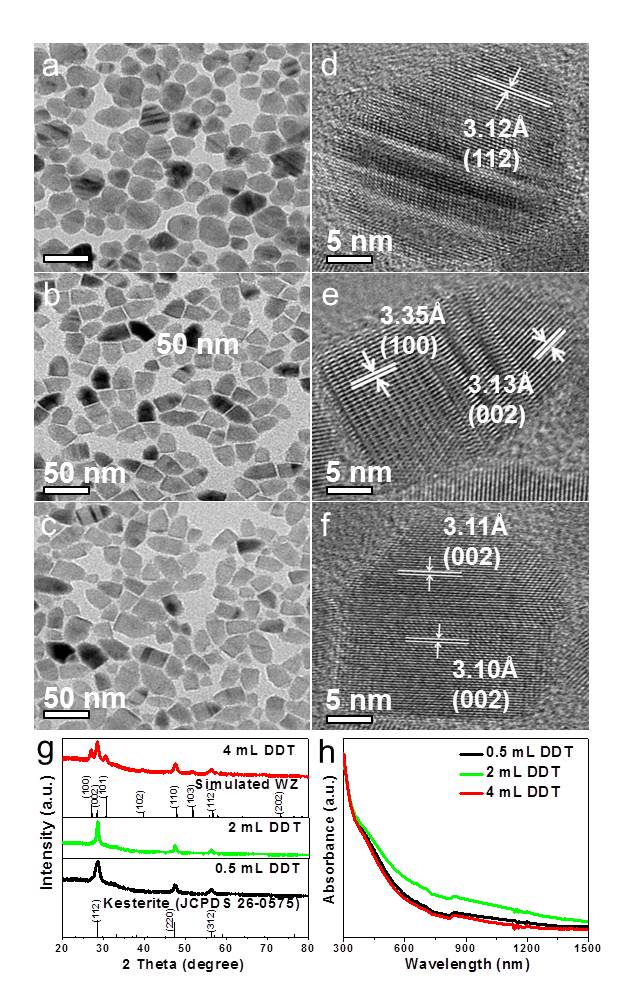
Fig. 2(a-c) TEM and (d-f) HRTEM images illustrating the bullet-like CZTS NCs obtained at 220 oC for 30 min in presence of different amounts of DT: 0.5 mL (a,d), 2 mL (b,e) and 4 mL (c,f). XRD patterns (g) and optical spectra (h) of the corresponding CZTS NCs shown in a-c.
References
- Y. Zou, X. Su and J. Jiang, J. Am. Chem. Soc., 2013, 135, 18377-18384.
- Z. Li, A. L. K. Lui, K. H. Lam, L. Xi and Y. M. Lam, Inorg. Chem., 2014, 53, 10874-10880.
- P. An, Z. Liang, X. Xu, X. Wang, H. Jin, N. Wang, J. Wang and F. Zhu, RSC Adv., 2015, 5, 6879-6885.
- X. Lu, Z. Zhuang, Q. Peng and Y. Li, Chem. Commun., 2011, 47, 3141-3143.
- C. Coughlan and K. M. Ryan, Cryst. Eng. Comm., 2015, 17, 6914-6922.
