Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Microbiology
1. Introduction
Perennial ryegrass (Lolium perenne L.) is used for forage in temperate regions throughout the world including Northern Europe, Pacific North West of USA, Japan, South-eastern Australia, and New Zealand [1,2,3,4,5]. It is the most commonly utilized pasture grass on dairy farms in Australia and has a high economic importance [6]. The asexual form of the endophytic fungus E. festucae var. lolii (previously known as Neotyphodium lolii and Acremonium lolii) is known to establish a symbiotic relationship with perennial ryegrass [7]. These interactions are beneficial to the pastoral agriculture industry as compounds produced by the endophyte confer resistance to biotic and abiotic stresses. For example, a select number of indole-diterpene class compounds, such the lolitrems A, B, and E that are present in endophyte-infected ryegrasses, are toxic to the larvae of the Argentine stem weevil (Listronotus bonariensis) [8,9]. The grass–endophyte association also produces secondary metabolites that are detrimental to grazing animals. The major toxins of concern are the alkaloids ergovaline and lolitrem B, which are present in old naturalized perennial ryegrass pastures containing the Standard Endophyte (SE) strain. Although both toxins are produced by endophyte-infected perennial ryegrass, ergovaline is normally most abundant in endophyte-infested tall fescue grass and causes the vasoconstrictive conditions fescue foot or summer slump disease [10,11]. The indole-diterpenes are predominant in endophyte-infected perennial ryegrass and lolitrem B is the end-point of the complex indole-diterpene biochemical pathway (Figure 1) [12]. Many of the indole-diterpene class of compounds, particularly the lolitrems, are reported as anti-mammalian alkaloids that significantly affect animal health. In particular, lolitrem B has been identified as a causative agent for perennial ryegrass staggers disease, a nervous disorder, notably of sheep and cattle that causes tremors [12]. However, despite the prevalence of perennial ryegrass in various geographic locations the toxicity reports are generally limited. Neurological signs associated with ryegrass staggers disease has been reported in animals, particularly sheep, grazing on perennial ryegrass in Australia, New Zealand, as well as Pacific northwest of USA and Europe [13,14]. This imposes a negative impact on industry, particularly dairy, meat, and wool production involving grazing animals [15]. Thus, forage improvement programs have been involved in selecting novel endophytes that do not produce the known toxins; however, complex mixtures of the intermediate compounds are still present in marketed forage grasses.
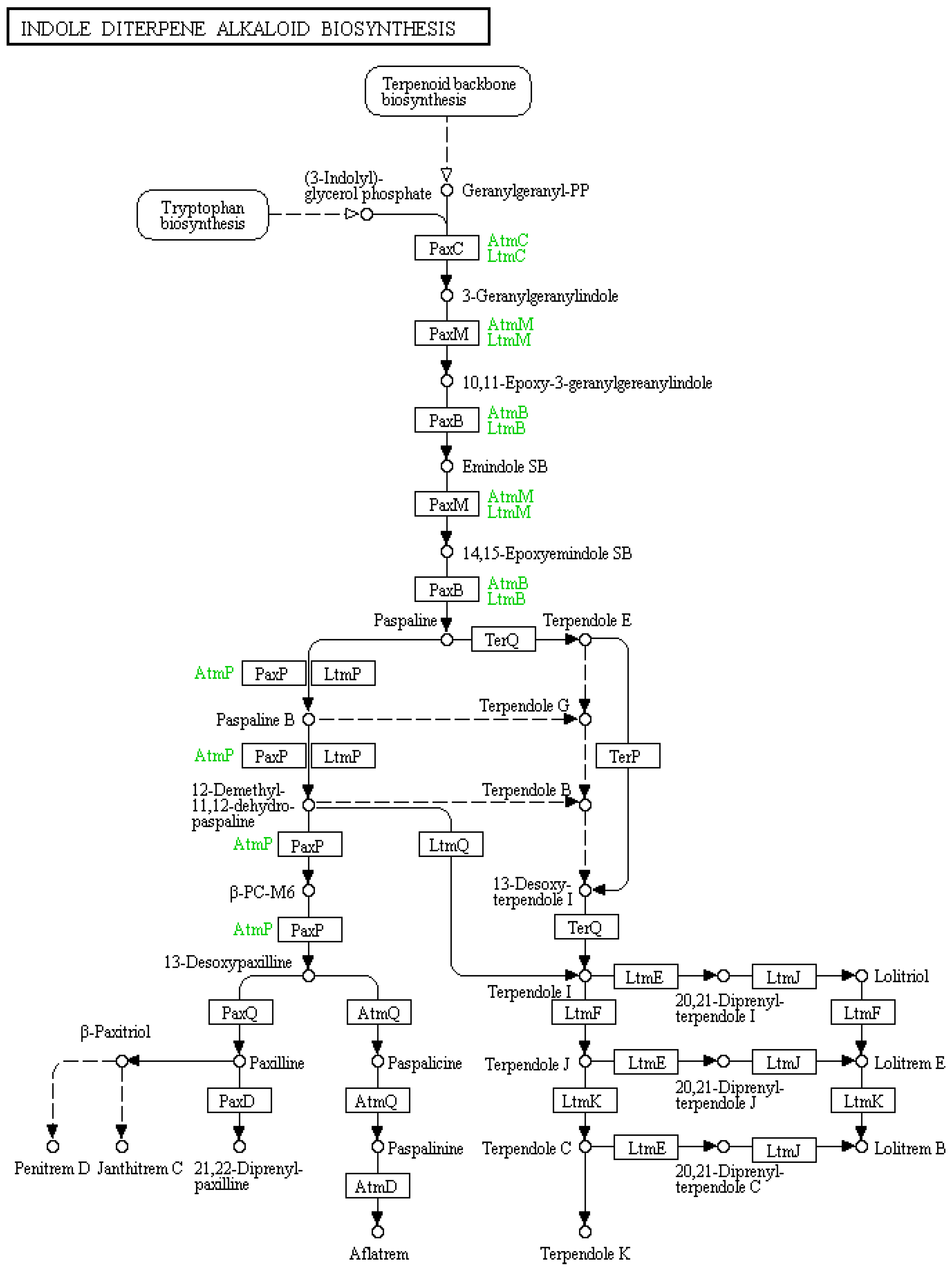
Figure 1. Indole-diterpene biosynthetic map sourced from KEGG pathways showing the enzymes responsible for producing indole-diterpene compounds at each step. Reproduced from [29,30,31].
References
- Forster, J.W.; Cogan, N.O.I.; Dobrowolski, M.P.; Francki, M.G.; Spangenberg, G.C.; Smith, K.F. Functionally associated molecular genetic markers for temperate pasture plant improvement. In Plant Genotyping II: SNP Technology; Henry, R.J., Ed.; CABI: Wallingford, UK, 2008; pp. 154–186.
- Young, C.A.; Hume, D.E.; McCulley, R.L. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Sci. 2013, 91, 2379–2394.
- Cunningham, P.J.; Foot, J.Z.; Reed, K.F.M. Perennial ryegrass (Lolium perenne) endophyte (Acremonium lolii) relationships: the Australian experience. Agric. Ecosyst. Environ. 1993, 44, 157–168.
- Tian, P.; Le, T.N.; Ludlow, E.J.; Smith, K.F.; Forster, J.W.; Guthridge, K.M.; Spangenberg, G.C. Characterisation of novel perennial ryegrass host-Neotyphodium endophyte associations. Crop Pasture Sci.2013, 64, 716–725.
- Van Zijll De Jong, E.; Dobrowolski, M.P.; Bannan, N.R.; Stewart, A.V.; Smith, K.F.; Spangenberg, G.C.; Forster, J.W. Global genetic diversity of the perennial ryegrass fungal endophyte Neotyphodium lolii. Crop Sci. 2008, 48, 1487–1501.
- Moate, P.J.; Williams, S.R.O.; Grainger, C.; Hannah, M.C.; Mapleson, D.; Auldist, M.J.; Greenwood, J.S.; Popay, A.J.; Hume, D.E.; Mace, W.J.; et al. Effects of wild-type, AR1 and AR37 endophyte-infected perennial ryegrass on dairy production in Victoria, Australia. Anim. Prod. Sci. 2012, 52, 1117–1130.
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White Jr, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215.
- Popay, A.J.; Hume, D.E.; Mainland, R.A.; Saunders, C.J. Field resistance to Argentine stem weevil (Listronotus bonariensis) in different ryegrass cultivars infected with an endophyte deficient in lolitrem B. New Zeal. J. Agr. Res. 1995, 38, 519–528.
- Prestidge, R.A.; Ball, O.J.P. The role of endophytes in alleviating plant biotic stress in New Zealand. In Proceedings of the Second International Symposium on Acremonium/Grass Interactions; Hume, D.E., Latch, G.C.M., Easton, H.S., Eds.; AgResearch: Palmerston North, New Zealand, 1993; pp. 141–151.
- Porter, J.K. Analysis of endophyte toxins: Fescue and other grasses toxic to livestock. J. Animal. Sci. 1995, 73, 871–880.
- Hovermale, J.T.; Craig, A.M. Correlation of ergovaline and lolitrem B levels in endophyte-infected perennial ryegrass (Lolium perenne). J. Vet. Diagn. Invest. 2001, 13, 323–327.
- Weedon, C.M.; Mantle, P.G. Paxilline biosynthesis by Acremonium loliae; a step towards defining the origin of lolitrem neurotoxins. Phytochemistry 1987, 26, 969–971.
- CHAPTER 14—Neurological Diseases. In Diagnostic Techniques in Equine Medicine, 2nd ed.; Taylor, F.G.R.; Brazil, T.J.; Hillyer, M.H. (Eds.) W.B. Saunders: Edinburgh, UK, 2009; pp. 287–304.
- CHAPTER 14—Diseases of the Nervous System. In Veterinary Medicine, 11th ed.; Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. (Eds.) W.B. Saunders: Edinburgh, UK, 2017; pp. 1155–1370.
- Kozák, L.; Szilágyi, Z.; Tóth, L.; Pócsi, I.; Molnár, I. Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: Biosynthetic diversity, threats and applications. Appl. Microbiol. Biotechnol. 2019, 103, 1599–1616.
- Gallagher, R.T.; Hawkes, A.D. The potent tremorgenic neurotoxins lolitrem B and aflatrem: A comparison of the tremor response in mice. Experientia 1986, 42, 823–825.
- Latch, G.C.M. Trichothecenes and other mycotoxins. In Proceedings of the International Mycotoxin Symposium, Sydney, Australia, August 1984.
- Plumlee, K.H.; Galey, F.D. Neurotoxic mycotoxins: A review of fungal toxins that cause neurological disease in large animals. J. Vet. Intern. Med. 1994, 8, 49–54.
- Thom, E.R.; Clark, D.A.; Waugh, C.D. Growth, persistence, and alkaloid levels of endophyte-infected and endophyte-free ryegrass pastures grazed by dairy cows in northern New Zealand. New Zeal. J. Agr. Res.1999, 42, 241–253.
- Miles, C.O.; Munday, S.C.; Wilkins, A.L.; Ede, R.M.; Towers, N.R. Large-scale isolation of lolitrem B and structure determination of lolitrem E. J. Agric. Food Chem. 1994, 42, 1488–1492.
- Russell, C.A. Letter: “Rye grass staggers”. Vet. Rec. 1975, 97, 295.
- Young, C.A.; Tapper, B.A.; May, K.; Moon, C.D.; Schardl, C.L.; Scott, B. Indole-diterpene biosynthetic capability of Epichloë endophytes as predicted by ltm gene analysis. Appl. Environ. Microbiol. 2009, 75, 2200–2211.
- Lewis, P.R.; Donoghue, M.B.; Cook, L.; Granger, L.V.; Hocking, A.D. Tremor syndrome associated with a fungal toxin: sequelae of food contamination. Med. J. Aust. 2005, 182, 582–584.
- Evans, T.J.; Gupta, R.C. Chapter 74—Tremorgenic Mycotoxins. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Amsterdam, Netherlands, 2018; pp. 1033–1041.
- Bluett, S.J.; Thom, E.R.; Clark, D.A.; Macdonald, K.A.; Minneé, E.M.K. Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zeal. J. Agr. Res. 2005, 48, 197–212.
- Reddy, P.; Deseo, M.A.; Ezernieks, V.; Guthridge, K.; Spangenberg, G.; Rochfort, S. Toxic indole diterpenes from endophyte-infected perennial ryegrass Lolium perenne L.: Isolation and stability. Toxins 2019, 11, 16.
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O. Isolation of lolicine A, lolicine B, lolitriol, and lolitrem N from Lolium perenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-epilolitrem N and 31-epilolitrem F. J. Agric. Food Chem. 1998, 46, 590–598.
- Munday-Finch, S. Aspects of the chemistry and toxicology of indole-diterpenoid mycotoxins involved in tremorganic disorder of livestock. Mycotoxin Res. 1997, 13, 88.
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic. Acids. Res. 2017, 45, D353–D361.
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids. Res. 2000, 28, 27–30.
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic. Acids. Res. 2019, 47, D590–D595.
- Gallagher, R.T.; Campbell, A.G.; Hawkes, A.D.; Holland, P.T.; McGaveston, D.A.; Pansier, E.A. Ryegrass staggers: The presence of lolitrem neurotoxins in perennial ryegrass seed. N. Z. Vet. J. 1982, 30, 183–184.
- McLeay, L.M.; Smith, B.L.; Munday-Finch, S.C. Tremorgenic mycotoxins paxilline, penitrem and lolitrem B, the nontremorgenic 31-epilolitrem B and electromyographic activity of the reticulum and rumen of sheep. Res. Vet. Sci. 1999, 66, 119–127.
- Dalziel, J.E.; Dunlop, J.; Finch, S.C.; Wong, S.S. Immune Response Inhibition Using Indole Diterpene Compound. WO2006115423A1, 2006. Available online: https://patents.google.com/patent/WO2006115423A1/ko (accessed on 2 November 2018).
- Dalziel, J.E.; Finch, S.C.; Dunlop, J. The fungal neurotoxin lolitrem B inhibits the function of human large conductance calcium-activated potassium channels. Toxicol. Lett. 2005, 155, 421–426.
- Knaus, H.-G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.H.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P.; Smith, A.B.; et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828.
- Imlach, W.L.; Finch, S.C.; Dunlop, J.; Meredith, A.L.; Aldrich, R.W.; Dalziel, J.E. The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J. Pharmacol. Exp. Ther. 2008, 327, 657–664.
- McMillan, L.K.; Carr, R.L.; Young, C.A.; Astin, J.W.; Lowe, R.G.; Parker, E.J.; Jameson, G.B.; Finch, S.C.; Miles, C.O.; McManus, O.B.; et al. Molecular analysis of two cytochrome P450 monooxygenase genes required for paxilline biosynthesis in Penicillium paxilli, and effects of paxilline intermediates on mammalian maxi-K ion channels. Mol. Genet. Genomics. 2003, 270, 9–23.
- Imlach, W.L.; Finch, S.C.; Zhang, Y.; Dunlop, J.; Dalziel, J.E. Mechanism of action of lolitrem B, a fungal endophyte derived toxin that inhibits BK large conductance Ca2+-activated K+ channels. Toxicon 2011, 57, 686–694.
