This entry summarizes most updated data on dry needling for mechanical neck pain disorders.
- dry needling, neck pain
1. Introduction
Neck pain is a musculoskeletal condition that often becomes chronic and can result in high levels of disability. The point prevalence is estimated to be 20%, whereas the lifetime prevalence can reach up to 70% in the general population [1]. The Global Burden of Disease Study identified neck pain as the fourth highest condition on number of years lived with disability [2]. Physical therapy is usually the first therapeutic option requested by individuals with neck pain. Several interventions, including cervical manual therapy [3], exercises [4], and education [5], have shown to be effective for the management of neck pain. Clinical practice guidelines for physical therapy management of neck pain recommend manual therapies combined with exercises as the therapeutic strategy for the proper management of these patients [6][7]. Further, clinical practice guidelines do not recommend other treatments, such as dry needling, not because there is evidence against the particular intervention but, rather, there is a lack of studies examining its use.
The etiology of mechanical neck pain is under debate, and it seems to be multifactorial. Some authors proposed that myofascial trigger points (TrPs) can play a role in neck pain development [8]. Simons et al. [8] defined a TrP as “a hypersensitive spot located in a taut band of skeletal muscle which stimulation induces referred pain symptoms and motor phenomena”. There is evidence showing that the referred pain elicited by active TrPs from neck musculature reproduces neck pain symptoms of insidious or traumatic origin [8]. Chiarotto et al. [9] found that TrPs in the upper trapezius is the most common finding in individuals suffering from neck pain.
Among the several approaches proposed for the treatment of TrPs, dry needling has received particular attention in the last decades [8][10]. Dry needling is defined as a “skilled intervention using a thin filiform needle to penetrate the skin that stimulates myofascial TrPs, muscles, and connective tissue for the treatment of musculoskeletal pain disorders” [11].
A few previous reviews have investigated the effectiveness of dry needling for inactivating TrPs associated with neck pain. Cagnie et al. concluded that dry needling can be recommended for upper trapezius muscle TrPs treatment; however, no quantitative analysis was conducted [12]. Liu et al. concluded that TrP dry needling could be recommended for the management of neck/shoulder pain of myofascial origin at short and mid-term follow-ups [13]. This meta-analysis only included pain intensity as the outcome and considered one month as a mid-term follow-up [13]. In addition, a greater number of randomized clinical trials investigating the effectiveness of dry needling in patients with TrPs associated to neck pain symptoms have been published after the Liu et al. meta-analysis [13].
2. Dry Needling and Neck Pain Intensity
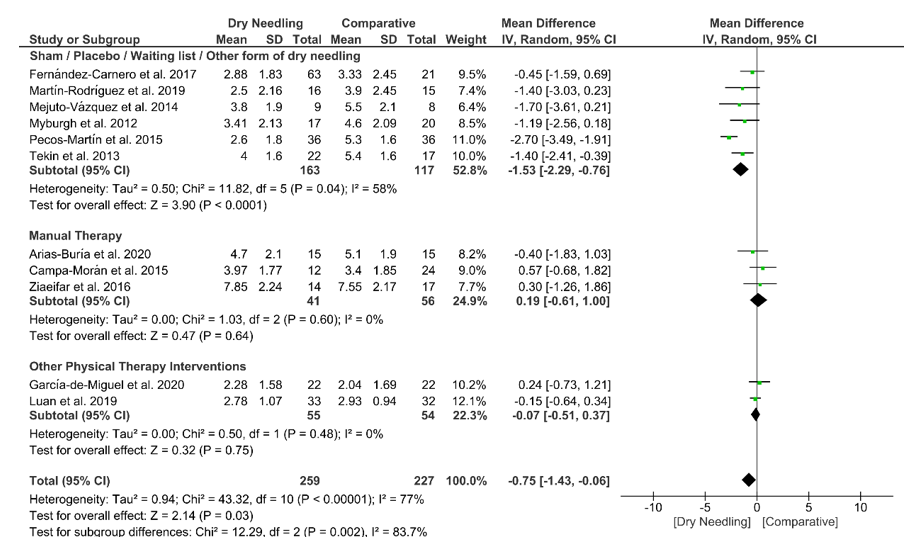
Dry needling exhibited a small overall significant effect (MD −0.75, 95% CI −1.43 to −0.06; p = 0.03 Z = 2.14, N = 486, n = 11 trials) for reducing neck pain immediately after the intervention vs. a comparison group but with substantial heterogeneity (I2 = 77%) between the trials (Figure 3). A significant effect (MD −1.53, 95% CI −2.29 to −0.76, p < 0.001) was found for the grouping analysis (p = 0.002) being significant comparing dry needling vs. sham/placebo/waiting list/other forms of dry needling (MD −1.53, 95% CI −2.29 to −0.76, p = 0.04). The funnel plot did not present potential publication bias (Figure S1).
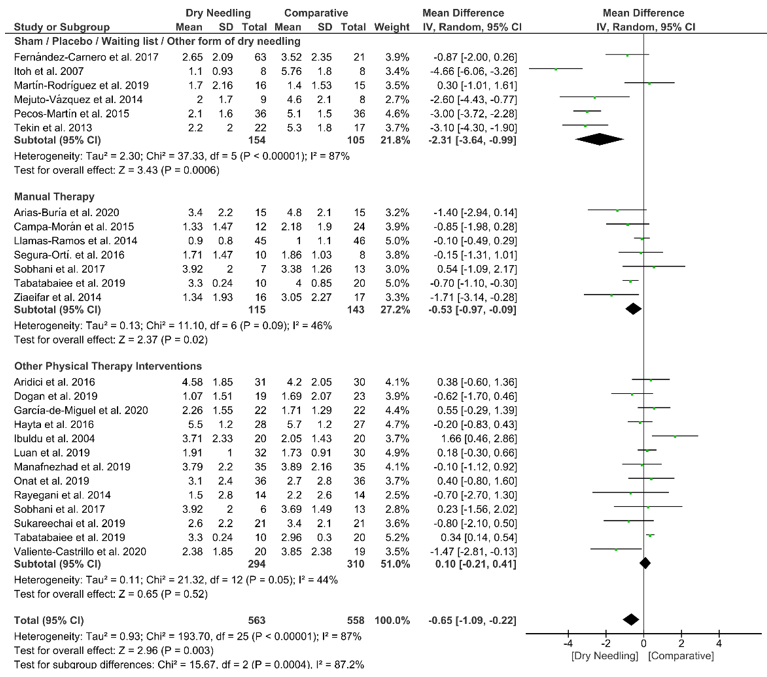
Dry needling also showed a significant overall short-term effect (MD −0.65, 95% CI −1.09 to −0.22; p = 0.003, Z = 2.96, N = 1121, n = 24 trials) for reducing the intensity of neck pain as compared to a comparative group but, also, with considerable heterogeneity (I2 = 87%) between the trials (Figure 4). Significant subgroup differences (p = 0.0004, I2 = 87.2%) were observed when comparing dry needling with sham/placebo/waiting list/other forms of dry needling (MD −2.31, 95% CI −3.64 to −0.99, p < 0.001) and with manual therapy (MD −0.53, 95% CI −0.97 to −0.09, p = 0.02), but not when comparing with other physical therapy interventions (MD 0.10, 95% CI −0.21 to 0.41, p = 0.52). The funnel plot did not present a potential publication bias (Figure S2).
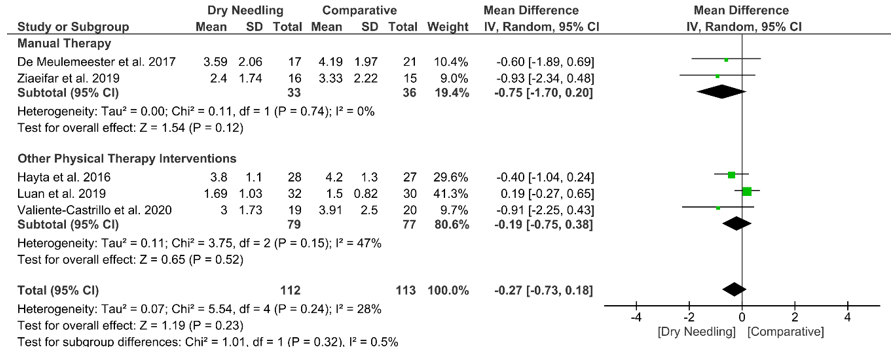
At mid-term, dry needling did not exhibit a significant overall effect (MD −0.27, 95% CI −0.73 to 0.18, p = 0.23, Z = 1.19, N = 225, n = 5 trials) for decreasing neck pain intensity when compared with a comparative group, with no significant heterogeneity (I2 = 28%) between the studies (Figure 5). No significant subgroup differences (p = 0.32, I2 = 0.5%) were observed. Table S1 summarizes the main results of the included studies.
Figure 3. Mean differences (MD) comparing the immediate effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on pain intensity.
Figure 4. Mean differences (MD) comparing the short-term effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy. SD:standard deviation; CI: confidence interval.
Figure 5. Mean differences (MD) comparing the mid-term effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy. SD:standard deviation; CI: confidence interval.
3. Dry Needling and Pain-Related Disability
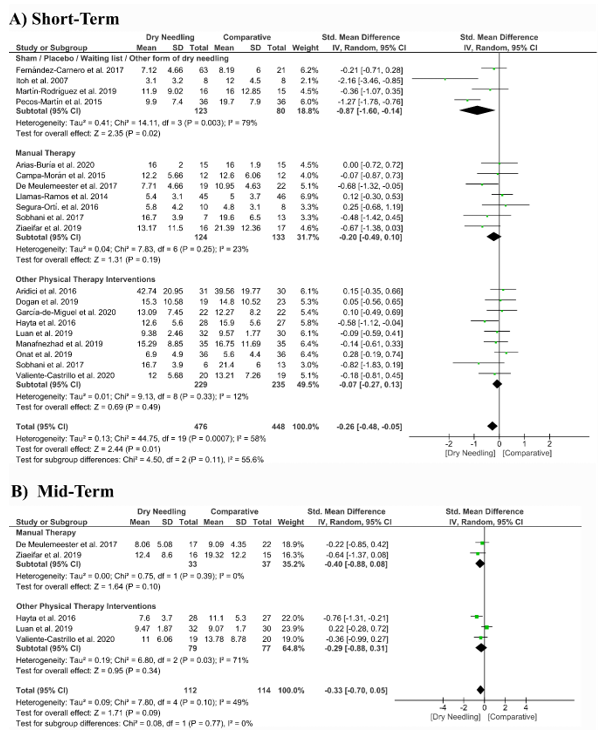
Dry needling had a significant overall small effect size (SMD −0.26, 95% CI −0.48 to −0.05, p = 0.001, Z = 2.44, N = 924, n = 20 trials) for improving pain-related disability at the short-term when compared with a comparative group but with moderate heterogeneity (I2 = 58%) among trials (Figure 6A). Significant differences were found when comparing dry needing with sham/placebo/waiting list/other forms of dry needling (SMD −0.87, 95% CI −1.60 to −0.14, p = 0.003) but not when compared with manual therapy (SMD −0.20, 95% CI −0.49 to 0.10, p = 0.19) or other physical therapy interventions (SMD −0.07, 95% CI −0.27 to 0.13, p = 0.49). The funnel plot presented asymmetry and publication bias (Supplementary Figure S3).
At mid-term follow-up, dry needling did not exhibit a significant overall effect (SMD −0.33, 95% CI −0.70 to 0.05, p = 0.09, Z = 1.71, N = 226, n = 5 trials) for reducing pain related-disability as compared to a comparative group, with moderate heterogeneity (I2 = 49%) among the trials (Figure 6B). No significant subgroup differences were found (p = 0.77, I2 = 0%). Table S1 summarizes the main results of the included studies.
Figure 6. Standardized mean differences (SMD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on pain-related disability at the (A) short- and (B) mid-terms. SD:standard deviation; CI: confidence interval.
4. Dry Needling and Pressure Pain Sensitivity (Pressure Pain Thresholds)
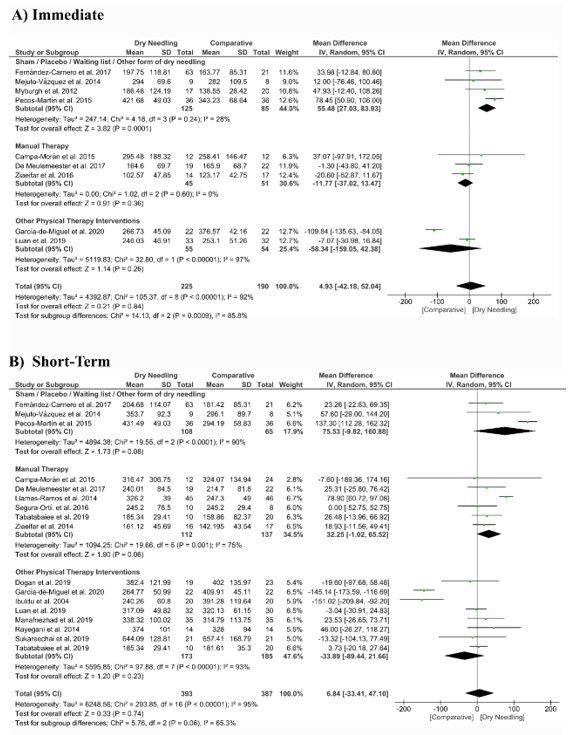
Dry needling did not show a significant overall effect immediately after (MD 4.93 kPa, 95% CI −42.18 to 52.04, n = 415, Z = 0.21, p = 0.84, Figure 7A) and at short-term (MD 6.84 kPa, 95% CI −33.41 to 47.10, n = 780, Z = 0.33, p = 0.74, Figure 7B) for increasing the pressure pain thresholds vs. a comparative group. The funnel plot did not present a potential publication bias (Supplementary Figure S4).
The analysis also revealed considerable heterogeneity (I2 > 95%) between the studies. Only the subgroup comparing dry needling with sham/placebo/waiting list/other forms of dry needling had a significant immediate effect (MD 55.48 kPa, 95% CI 27.03 to 83.93, p < 0.001, Figure 7A).
Figure 7. Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the pressure pain thresholds (kPa) (A) immediately after and (B) at the short-term. SD:standard deviation; CI: confidence interval.
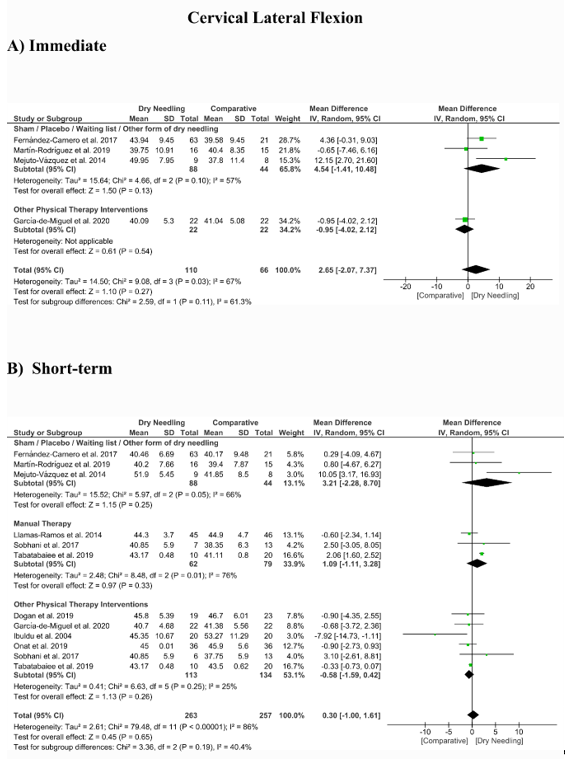
5. Dry Needling and Cervical Range of Motion
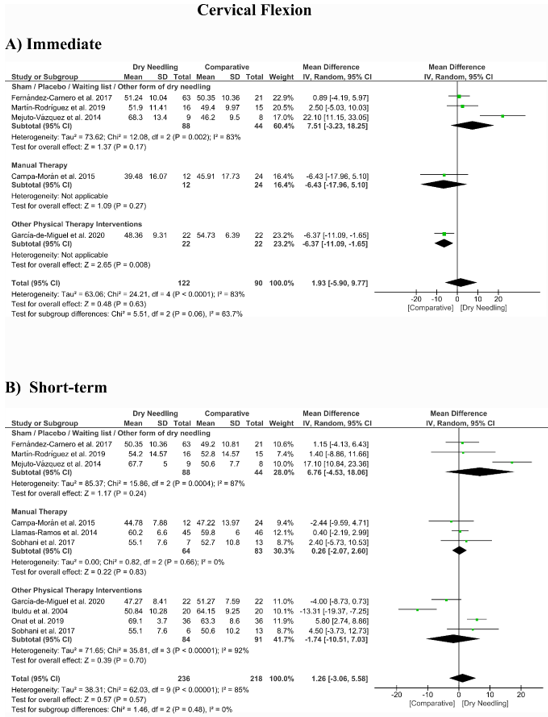
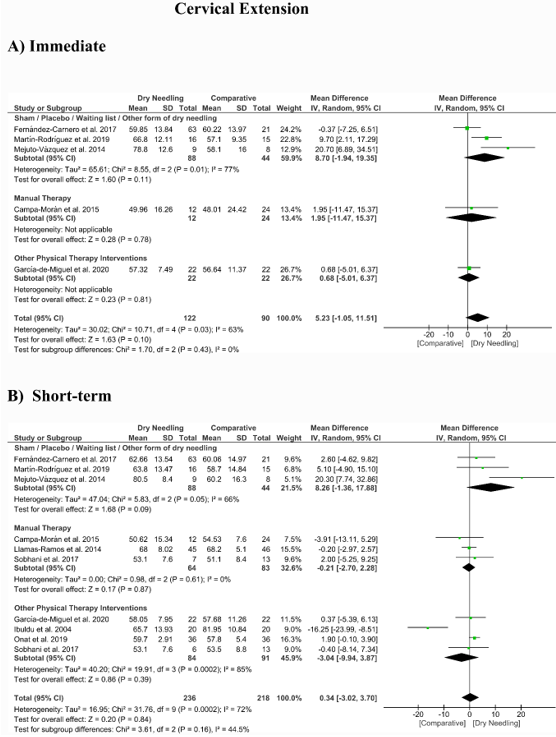
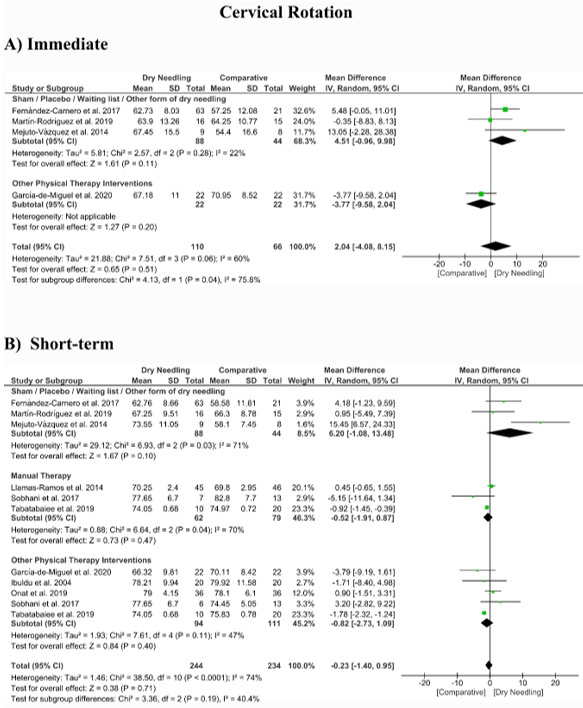
No significant overall effects of dry needling immediately after on the cervical range of motion when compared with a comparison group were observed: flexion (MD 1.93°, 95% CI −5.90° to 9.77°, n = 212, Z = 0.48, p = 0.63, Figure 8A), extension (MD 5.23°, 95% CI −1.05° to 11.51°, n = 212, Z = 1.63, p = 0.10, Figure 9A), rotation (MD 2.04°, 95% CI −4.08° to 8.15°, n = 176, Z = 0.65, p = 0.51, Figure 10A), and lateral-flexion (MD 2.65°, 95% CI −2.07° to 7.37°, n = 176, Z = 1.10, p = 0.27, Figure 11A). Similarly, no significant overall short-term effect of dry needling on cervical flexion (MD 1.26°, 95% CI −3.06° to 5.58°, n = 458, Z = 0.57, p = 0.57, Figure 8B), extension (MD 0.34°, 95% CI −3.02° to 3.70°, n = 454, Z = 0.20, p = 0.84, Figure 9B), rotation (MD −0.23°, 95% CI −1.40° to 0.95°, n = 478, Z = 0.38, p = 0.71, Figure 10B), and lateral-flexion (MD 0.30°, 95% CI −1.00° to 1.61°, n = 520, Z = 0.45, p = 0.65, Figure 11B) was found. All group analyses showed substantial heterogeneity. Table 3 summarizes the main results of the included studies.
Figure 8. Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in flexion (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 9. Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in extension (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 10. Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in rotation (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
Figure 11. Mean differences (MD) comparing the effects of dry needling alone against sham/placebo/waiting list/other forms of dry needling or manual therapy or other physical therapy interventions on the cervical range of motion in lateral-flexion (A) immediately after and (B) at the short-term. SD: standard deviation; CI: confidence interval.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9103300
References
- Hoy, D.; March, L.; Woolf, A.; Blyth, F.; Brooks, P.; Smith, E. The global burden of neck pain: Estimates from the global burden of disease 2010 study. Rheum. Dis. 2014, 73, 1309–1315.
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Gross, A.; Langevin, P.; Burnie, S.J.; Bédard-Brochu, M.; Empey, B.; Dugas, E.; Faber-Dobrescu, M.; Andres, C.; Graham, N.; Goldsmith, C.H. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst. Rev. 2015, 9, CD004249.
- Gross, A.; Kay, T.M.; Paquin, J.; Blanchette, S.; Lalonde, P.; Christie, T.; Dupont, G.; Graham, N.; Burnie, S.J.; Gelley, G. Exercises for mechanical neck disorders. Cochrane Database Syst. Rev. 2015, 1, CD004250.
- Gross, A.; Forget, M.; St George, K.; Fraser, M.M.H.; Graham, N.; Perry, L.; Burnie, S.J.; Goldsmith, C.H.; Haines, T.; Brunarski, D. Patient education for neck pain. Cochrane Database Syst. Rev. 2012, 3, CD005106.
- Bier, J.D.; Scholten-Peeters, W.G.M.; Staal, J.B.; Pool, J.; van Tulder, M.W.; Beekman, E.; Knoop, J.; Meerhoff, G.; Verhagen, A.P. Clinical practice guideline for physical therapy assessment and treatment in patients with nonspecific neck pain. Ther. 2018, 98, 162–171.
- Blanpied, P.R.; Gross, A.R.; Elliott, J.M.; Devaney, L.L.; Clewley, D.; Walton, D.M.; Sparks, C.; Robertson, E.K.; Altman, R.D.; Beattie, P. Neck pain: Revision 2017: Clinical practice guidelines linked to the international classification of functioning, disability and health from the orthopaedic section of the American Physical Therapy Association. Orthop. Sport. Phys. Ther. 2017, 47, A1–A83.
- Simons, D.G.; Travell, J.G. Myofascial Pain and Dysfunction: The Trigger Point Manual, 3rd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019.
- Chiarotto, A.; Clijsen, R.; Fernandez-De-Las-Penas, C.; Barbero, M. Prevalence of myofascial trigger points in spinal disorders: A systematic review and meta-analysis. Phys. Med. Rehabil. 2016, 97, 316–337.
- Dommerholt, J.; Fernandez-de-las Peñas, C. Trigger Point Dry Needling: An Evidence and Clinical-Based Approach, 2nd ed.; Elsevier: London, UK, 2019.
- Description of Dry Needling in Clinical Practice: An educAtional Resource Paper; APTA: Alexandria, VA, USA, 2013.
- Cagnie, B.; Castelein, B.; Pollie, F.; Steelant, L.; Verhoeyen, H.; Cools, A. Evidence for the use of ischemic compression and dry needling in the management of trigger points of the upper trapezius in patients with neck pain: A systematic review. J. Phys. Med. Rehabil. 2015, 94, 573–583.
- Liu, L.; Huang, Q.-M.; Liu, Q.-G.; Ye, G.; Bo, C.-Z.; Chen, M.-J.; Li, P. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: A systematic review and meta-analysis. Phys. Med. Rehabil. 2015, 96, 944–955.