Severe burn injuries remain a major health problem due to high rates of mortality, residual morbidity, and/or aesthetic damages. To find new therapies aimed at promoting a harmonious healing of skin burns, it is important to develop models which take into account the unique properties of the human skin. Based on previously described models of burn injury performed on human skin explants, we hypothesized that maintaining explants under constant tension forces would allow to more closely reproduce the pathophysiological processes of skin remodeling. We thus. Here, we set up and characterized an improved model of deep second-degree burn injury on ex vivo cultured human skin explants at air-liquid interface and maintained under conditions of constant tension forces. A spontaneous re-epithelialization of the lesion was observed 8 to 9 days post burn and was found to rely on the proliferation of basal keratinocytes at the wound edges. Collagen VII at the dermo-epidermal junction reformed along with the progression of re-epithelializatio and a synthesis of procollagen III was observed in the dermis at the wound site. These findings indicate that our model is suitable for the assessment of clinically-relevant therapies aimed at modulating the kinetics of re-epithelialization and/or the activation of fibroblasts following skin burn injuries. In this regard, we evaluated the use of a thermoreversible poloxamer hydrogel as a vehicle for topically-testable therapeutic molecules. Our data showed that, although useful for drug formulation, the p407/p188 poloxamer hydrogel induces a delay of skin re-epithelialization in humans skin explants submitted to experimental burn injury.
- burn
- human skin
- ex vivo
- wound healing
- poloxamer hydrogel
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition: Severe burn injuries remain a major health problem due to high rates of mortality, residual morbidity, and/or aesthetic damages. To find new therapies aimed at promoting a harmonious healing of skin burns, it is important to develop models which take into account the unique properties of the human skin.
Content
Histological Characterization of Ex Vivo Cultured Human Skin Explants
In order to optimize our protocol of ex vivo cultured skin explants grown at the air–liquid interface, we compared two different culture media (Figure 1) with regard to the general skin histological structure.
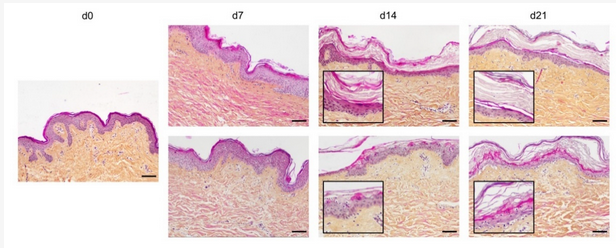
Figure 1. Assessment of culture media impact on ex vivo cultured human skin explants. Hematoxylin phloxine saffron (HPS) staining was performed on human skin explants harvested at different time points of the culture. Results obtained with two distinct culture media were compared: medium A (standard culture medium; upper panels) and medium B (medium used for three-dimensional (3D) cultures of skin cells; lower panels). From day 14 onward, skin explants cultured in medium B exhibited a detachment of the epidermis (bottom panels—days 14 and 21). Results obtained for each condition are representative of three series of experiments performed on skin explants derived from three donors. Scale bar: 100 µm.
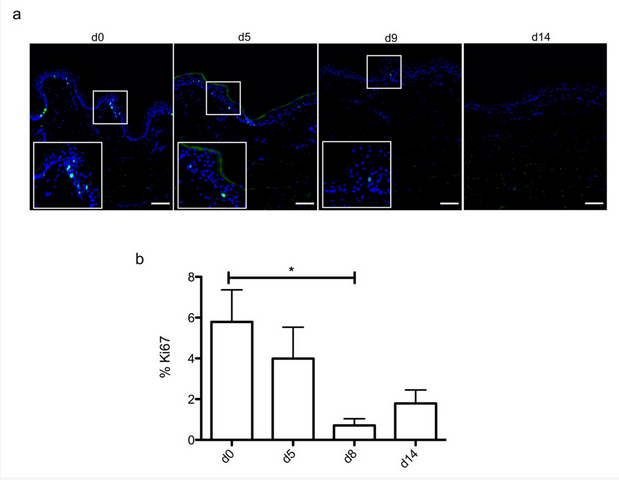
Figure 2. Demonstration of a persisting proliferating potential in the basal keratinocytes of cultured human skin explants. (a) Immunostaining of the proliferation marker Ki67 was performed on human skin explants harvested at different time points of the culture. Ki67+ keratinocytes were observed in the basal layer in freshly isolated skin explants (day 0) and were still detectable on day 5 following culture initiation. Only a few Ki67+ cells remained detectable in the basal layer of skin explants from day 9 onward. (b) The proliferation index of keratinocytes (ratio of proliferating cells relative to the total number of epidermal cells) was measured in human skin explants harvested at different time points of the culture. For each donor (n = 3) and for each time point, measurements were carried out on three distinct areas and expressed as mean values. Statistical significance was assessed with the paired t-test. *: p < 0.05. Scale bar: 100 µm.
Setting up of an Experimental Model of a Deep Second-Degree Burn Injury on Ex Vivo Cultured Human Skin Explants
A metal rod heated to 100 °C was applied for different time periods (1 s, 5 s, and 10 s) on ex vivo cultured human skin explants (Figure 3). HPS staining was then performed on burnt areas at 10 min post burn, and on days 7, 14, and 21. We observed that a 1 s long application impacted only the superficial epidermal layers without damaging the basal layer (days 0 and 7) and was followed by a full re-epithelialization (day 14), which corresponds to a model of first-degree burn. In contrast, a 5 s or 10 s long application of the heated metal rod induced lesions of the whole epidermis layers as assessed by HPS staining. More specifically, we found that, 10 min after experimental burns were performed, epidermal cells were vacuolized throughout all epidermal layers and the epidermis was detached from the dermis. On day 7, following the debridement step performed on day 1, the burnt area was devoid of epidermis, which corresponds to the histological features of a deep second-degree burn. Following either 5 s or 10 s long experimental burns, the epidermis was fully re-epithelialized on day 14 post burn. This re-epithelialized epidermis comprised all the epidermal layers up to the stratum corneum (Figure 3). Since burns of longer durations are more likely to also impact the dermis, we chose to further assess our model under conditions of 10 s long experimental burns.
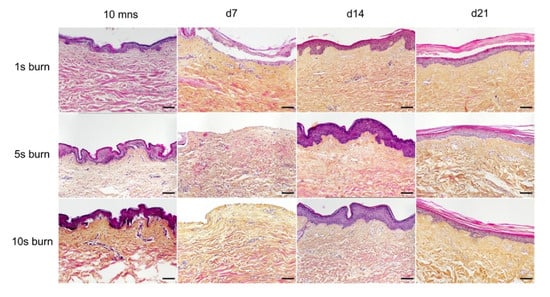
Figure 3. Assessment of burn duration impact on epidermal integrity in ex vivo cultured human skin explants. Hematoxylin phloxine saffron (HPS) staining was performed on human skin explants harvested at different time points post burn (10 min and days 7, 14, and 21). Burns were generated with a metal rod heated to 100 °C and applied for 1 s (upper panels), 5 s (middle panels) or 10 s (bottom panels). A 1 s burn impacted only the superficial layers of the epidermis, whereas 5 s and 10 s burns induced alterations of the whole epidermis. Under the three conditions tested, the burnt area was fully re-epithelialized on day 14. Images were taken in the center of the burn. Results obtained for each condition are representative of three series of experiments performed on skin explants derived from three donors. Scale bar: 100 µm.
Kinetics of Re-Epithelialization in Human Skin Explants Subjected to Experimental Deep-Second Degree Burn Injury
To investigate the kinetics of re-epithelialization in our model of experimental burn injury, serial histological analyses were performed on lesioned skin explant samples obtained from three distinct donors. The extent of re-epithelialization was assessed on days 0, 1, 3, 5, 6, 7, 8, 9, 10, and 14 post burn by HPS staining. Slight interindividual differences were observed regarding the time point from which re-epithelialization initiates (day 8 for two donors, day 9 for one donor). Numeration of Ki67+ proliferating keratinocytes was obtained on days 0, 5, 8, 9, and 14. However, the same series of events took place in the burnt area, as exemplified in Figure 4: (i) proliferating keratinocytes formed a monolayer of cells on a restricted area localized on the edges of the lesion (re-epithelialization, day 1); (ii) the whole lesioned area was then progressively colonized by a monolayer of proliferating keratinocytes (re-epithelialization, days 2–4); (iii) the epidermis progressively thickened from the edges to the center of lesions, leading eventually to the formation of a stratum corneum layer (re-epithelialization, day 6). Of note, before re-epithelialization of the injured skin initiated, the percentage of proliferating keratinocytes increased on day 5 post burn in the non-lesioned areas adjacent to the lesion edges (Figure 4c). Interestingly, when a hair follicle could be observed in such adjacent non-lesioned areas, we could also demonstrate the presence of Ki67 proliferating cells which appeared to form a migratory stream from hair follicles toward the lesion edges (Figure 4d). Initiation of re-epithelialization was paralleled by a marked increase in keratinocyte proliferation index which reached 56% at the wound margins (Figure 4b,c). Such an index then decreased over time along with the progression of re-epithelialization from the edges to center of lesions and in parallel with the progressive thickening of the epidermis. Thus, on day 14 post burn, the keratinocyte proliferation index reached similar levels (10%) to that observed in non-lesioned skin explants on day 0 of the culture protocol (6%).
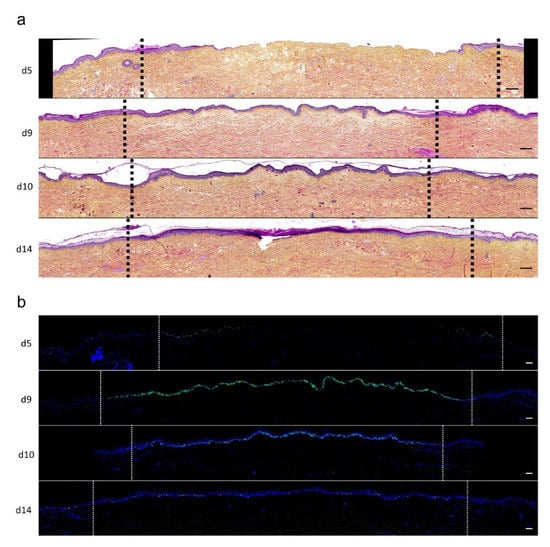
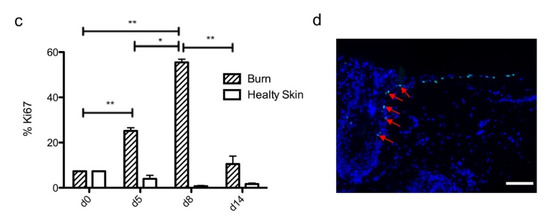
Figure 4. Kinetics of re-epithelialization from day 8 onward in ex vivo cultured human skin explants submitted to 10 s long experimental burn injury. Following a 10 s long experimental burn performed on ex vivo cultured human skin explants, the kinetics of re-epithelialization was assessed by HPS staining (a) and immunostaining of the proliferation marker Ki67 (b–d). (a) Wound closure was operated by proliferating keratinocytes which migrated from the wound edges and then formed an epidermal tongue on d5, before covering the whole lesioned area on day 8. A complete differentiation of the epidermis up to the formation of the superficial layers, including the stratum corneum, was then observed on day 14. (b,c) The keratinocyte proliferation index (ratio of proliferating Ki67+ cells relative to the total number of epidermal cells) was measured in human skin explants harvested at different time points of the culture. For each donor (n = 3) and for each time point, measurements were carried out on both edges of the lesion and expressed as mean values. On day 5 post burn, proliferating basal keratinocytes were only observed in the epidermal tongue, close to the wound margins. In this area, the keratinocyte proliferation index more than doubled as compared to the proliferation index observed in day 0 (control) skin explants (c). A peak in keratinocyte proliferation index was observed on day 8/9 with 55% of proliferating cells in the edges of the re-epithelialized lesion. Interestingly, several layers of Ki67+ epidermal cells were observed at this stage. Finally, on day 14 post burn, the keratinocyte index reached a mean of 10%, close to that observed in the epidermis of day 0 (control) skin explants (6%). (d) Demonstration of proliferating cells within the dermis in close vicinity to a hair follicle at the edge of the lesioned area (red arrows). Dashed lines in black (a) or white (b) delineate the burnt area from the adjacent non-lesioned skin areas. Statistical significance was assessed with the paired t-test. * p < 0.05, ** p < 0.01. Scale bar: 100 µm.
Kinetics of Dermal Remodeling in Human Skin Explants Subjected to Experimental Deep Second-Degree Burn Injury
In order to quantitatively and qualitatively assess dermal remodeling in our experimental model of deep second-degree burn injury, we performed serial immunostainings of collagen I, collagen III, procollagen III, and α-smooth muscle actin (αSMA), a marker of myofibroblasts, on skin explants on days 0, 5, 8, 10, and 14 post burn. While the levels and expression patterns of collagen I, collagen III, and αSMA appeared unchanged at the different time points studied (Figure S1, Supplementary Materials), we observed a marked decrease in procollagen III staining intensity starting on day 8 in the dermis underlying the burnt epidermal area (Figure 5). Although less pronounced, such a decreased staining intensity was still observed on day 10 post burn. On day 14, procollagen III staining intensity returned to basal levels and was comparable to day 0 staining. It should be noticed that, on day 5 post burn, we repeatedly found that human leukocyte antigen (HLA) class II positive cells appeared to accumulate in the wound margins in both the epidermis and dermis (Figure S2, Supplementary Materials). This observation, which may reflect a nonspecific activation of immune or nonimmune cells in particular blood vessels (Figure S2, Supplementary Materials), indicates that human skin explants are endowed with a certain level of immunocompetence, which may prove to be of interest in future studies.
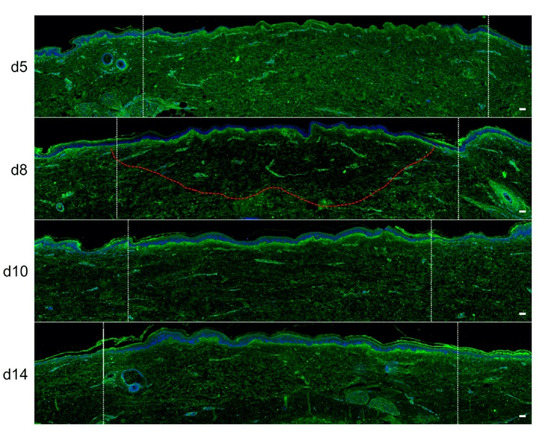
Figure 5. Kinetics of procollagen III synthesis in ex vivo cultured human skin explants submitted to 10 s long experimental injury. Immunostaining of procollagen III was performed on human skin explants harvested at different time points post burn (days 5, 8, 10, and 14). No significant change was observed on day 5. On day 8, a decreased intensity in procollagen III staining (area delimited by a red dotted line) was observed in the dermal area adjacent to the burnt epidermis. On day 10, this decrease in procollagen III staining intensity was less pronounced but still present until it returned to baseline levels on day 14. Experiments were performed on skin explants derived from three donors. White dashed lines delineate the burnt area. Scale bar: 100 µm.
Kinetics of Dermo-Epidermal Junction (DEJ) Restoration in Human Skin Explants Subjected to Experimental Deep Second-Degree Burn Injury
To assess the kinetics of DEJ regeneration in our model of experimental deep second-degree burn injury, a staining of collagen VII was carried out on days 5, 8, 10, and 14 post burn (Figure 6). Interestingly, while no collagen VII staining remained present at the DEJ on day 5 post burn, the process of re-epithelialization was temporally associated with a progressive restoration of the DEJ. Such a process could be demonstrated at the DEJ of newly formed monolayers of keratinocytes, in the lesion edges. However, the intensity of collagen VII staining appeared to also parallel the progressive increase in epidermis thickness, which similarly started in the lesion edges before extending toward the center of the burnt area (Figure 6a,b).
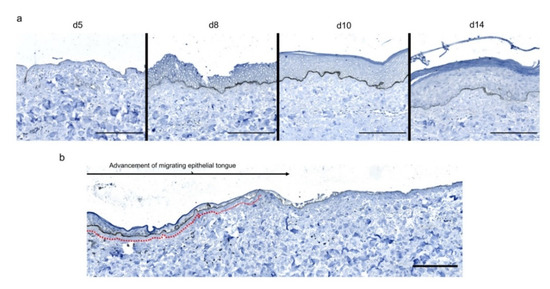
Figure 6. Kinetics of the dermo-epidermal junction (DEJ) repair in ex vivo cultured human skin explants submitted to 10 s long experimental burn injury. A 10 s burn was carried out on ex vivo cultured human skin explants and collagen VII immunostaining was performed on skin samples of the burn area harvested at different time points post burn (days 5, 8, 10, and 14. (a) In the center of the burnt area, no collagen VII remained detectable on day 5. However, in the same area, neosynthesized collagen VII was observed at the DEJ of the re-epithelialized epidermis on day 8 and onward. (b) In the wound margins, collagen VII staining was observed at the DEJ of the epidermal tongue formed by a few layers of cells. Experiments were performed on skin explants derived from three donors. Scale bar: 100 µm.
The Topical Application of a Poloxamer Hydrogel Delays but Does Not Prevent Tissue Repair in Human Skin Explants Subjected to Experimental Deep Second-Degree Burn Injury
We then sought to determine whether our experimental model could be suitable for the therapeutic assessment of topically applied regenerative compounds. To this goal, we assessed the impact of a poloxamer hydrogel which was previously shown to exhibit exploitable vehicle properties in the context of pharmacological tests [20,21,22]. Daily topical applications of such a poloxamer hydrogel were performed on areas of lesioned skin explants in our model of experimental second-degree burn injury. Serial HPS staining and immunolabeling of Ki67+ proliferating keratinocytes were then performed of skin explants on days 5, 8/9, 10, and 14 post burn (Figure 7b). Surprisingly, we constantly observed that initiation of re-epithelialization was delayed in hydrogel-treated skin explants as could be observed only on day 10 as compared to day 8 in untreated burnt explants (Figure 7a). However, a full re-epithelialization was achieved on day 14 in hydrogel-treated skin explants, indicating that, once re-epithelialization is initiated, tissue repair is otherwise unchanged by the topical application of a poloxamer hydrogel. Favoring this view, the proliferation index of keratinocytes in the lesioned area was delayed by roughly 2 days but followed a kinetics which was strictly similar to that observed in untreated skin explants submitted to experimental injury (Figure 7c). Thus, on day 5, 7% proliferating keratinocytes were observed at the edges of the gel-treated burn areas compared to 27% for untreated burns (Figure 7c). In the hydrogel-treated burn area, the percentage of proliferating cells reached 47% on day 8, which is similar to the proliferation index observed in untreated burnt explants (55%). On the other hand, on day 14, the hydrogel-treated burn still contained 33% proliferating keratinocytes compared to 10% in untreated burnt explants.
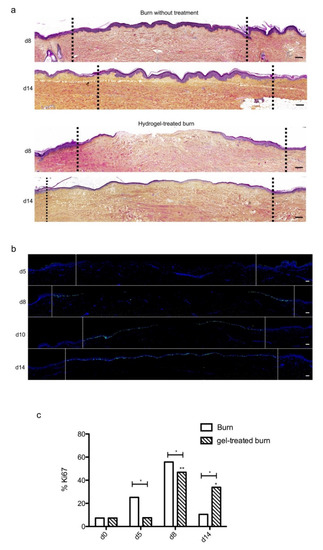
Figure 7. Kinetics of re-epithelialization in poloxamer hydrogel-treated ex vivo cultured human skin explants submitted to 10 s long experimental burn injury. A 10 s burn was carried out on ex vivo cultured human skin explants and, after wound debridement, on day 1, daily treatment with poloxamer p407/p188 hydrogel was applied. The impact of hydrogel applications on the healing process was assessed by (a) morphological analysis using HPS staining and (b) immunostaining of the proliferation marker Ki67. (a,b) On day 8 post burn, the hydrogel-treated lesion was not re-epithelialized, in contrast to the untreated lesion. The treated wound was re-epithelialized on day 14 but only 3–4 layers of keratinocytes were observed in the central burnt area, indicating that re-epithelialization was delayed by hydrogel treatment. (c) The keratinocyte proliferation index (ratio of Ki67+ proliferating cells relative to the total number of epidermal cells) was measured in human skin explants harvested at different time points of the culture. For each donor (n = 3) and for each time point, measurements were carried out on both edges of the lesion and expressed as mean values. No increase in proliferation index was noticed on day 5 in gel-treated lesions in contrast to the untreated lesions. On day 8, the proliferation index was similar in both conditions (47% in gel-treated lesions vs. 55% in untreated lesions). On day 14, the proliferation index in gel-treated burns was higher as compared to untreated burns (33% in gel-treated lesions vs. 6% in untreated lesions). Dashed lines in black (a) or white (b) delineate the burnt area. Statistical significance was assessed with the paired t-test. * p < 0.05, ** p < 0.01. Scale bar: 100 µm.
Topical treatment with poloxamer gel, thus, causes a delay in re-epithelialization and a delay in keratinocyte proliferation but does not prevent or qualitatively modify the process of epidermal tissue repair.
We also assessed the impact of poloxamer hydrogel on the dermal extracellular matrix remodeling and focused our analysis of the kinetics of procollagen III staining alterations (Figure 8). As observed with regard to re-epithelialization and keratinocyte proliferation index, we found that the synthesis of procollagen III followed a delayed kinetics of alterations in hydrogel-treated vs. untreated burnt explants. Such a delay may be estimated to reach 2 days and was responsible for obvious differences in terms of procollagen III staining intensities on day 10 post burn when comparing hydrogel-treated vs. untreated burnt explants. On day 14, however, the same amount of procollagen III was observed in the center of the burnt area irrespective of the application of a poloxamer hydrogel.
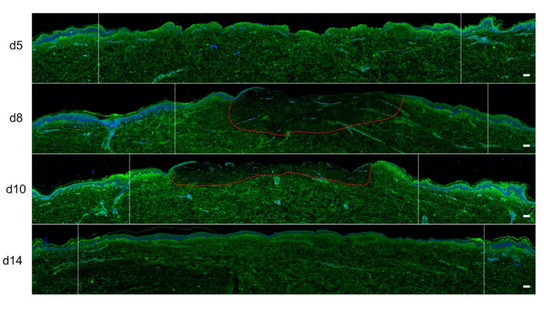
Figure 8. Kinetics of procollagen III synthesis in poloxamer hydrogel-treated ex vivo cultured human skin explants submitted to 10 s long experimental burn injury. Immunostaining of procollagen III was performed on gel-treated human skin explants harvested at different time points post burn (days 5, 8, 10, and 14). After wound debridement, on day 1, daily treatment with poloxamer p407/p188 hydrogel was applied. No significant change was observed on day 5 in the burn area compared to healthy skin. On day 8, a decreased intensity in procollagen III staining (area delimited by a red dotted line) was observed in the dermal area adjacent to the burnt epidermis. On day 10, this decrease in procollagen III staining intensity was less pronounced but still present until it returned to baseline levels on day 14. Experiments were performed on skin explants derived from three donors. White dashed lines delineate the burnt area. Scale bar: 100 µm.)
This entry is adapted from the peer-reviewed paper 10.3390/ijms21186956
