Epithelial ovarian cancer is a highly lethal gynecological malignancy that is characterized by the early development of disseminated metastasis. Though ovarian cancer has been generally considered to preferentially metastasize via direct transcoelomic dissemination instead of the hematogenous route, emerging evidence has indicated that the hematogenous spread of cancer cells plays a larger role in ovarian cancer metastasis than previously thought. Considering the distinctive biology of ovarian cancer, an in-depth understanding of the biological and molecular mechanisms that drive metastasis is critical for developing effective therapeutic strategies against this fatal disease. The recent “cancer stem cell theory” postulates that cancer stem cells are principally responsible for tumor initiation, metastasis, and chemotherapy resistance.
1. Introduction
Epithelial ovarian cancer is the most lethal cause of death among gynecological malignancies and is characterized by an early metastatic spread throughout the peritoneal cavity, along with extensive disseminated tumors, omental caking, and accumulation of malignant ascites [
1,
2]. The high mortality and low survival rate in ovarian cancer can be attributed to its nonspecific symptoms that generally manifest only after progression to an advanced stage, as well as the fact that only few women are diagnosed before cancer metastasis to the peritoneal cavity or distant parenchymal organs [
3,
4,
5]. Though most patients with the advanced stage of the disease may respond to a combination of taxane and platinum-based chemotherapy, chemoresistant residual cancer cells can persist in metastatic sites, where they remain dormant for prolonged periods after initial therapy and eventually lead to a relapse [
6,
7,
8]. Despite ongoing efforts in developing extirpative surgery and intensive combination chemotherapy, long-term clinical outcomes in patients with advanced ovarian cancer have not significantly improved over the recent decades [
9,
10,
11].
The cancer stem cell theory has been recently postulated and states that cancers are composed of hierarchies of cells sustained by tumor-initiating cells, conceptually termed cancer stem cells, with distinct phenotypes and a high tumorigenic potential [
12,
13]. Inherently, cancer stem cells possess the ability to self-renew and differentiate into multiple lineages of differentiated cancer cells, thereby establishing both a phenotypic and functional heterogeneity in the hierarchical organization of tumors [
14,
15]. Additionally, a large body of research indicates that a subpopulation of cancer stem cells is practically responsible for driving metastasis, therapy resistance, and the relapse of cancers [
16,
17]. In regard to ovarian cancer, Bapat et al. provided the first evidence for the existence of ovarian cancer stem cells in the malignant ascites in 2005; thereafter, extensive efforts have been made to evaluate the biological mechanisms that regulate ovarian cancer stemness [
18]. Clinically, ovarian cancer stem cells have been shown to survive conventional chemotherapy, which generally targets rapidly dividing cancer progenitor cells or more differentiated cancer cells. This small population of cancer stem cells then gives rise to chemoresistant recurrent tumors at metastatic sites [
9,
19]. Given the distinct biology of ovarian cancer stem cells, an elucidation of the cellular and molecular mechanisms underlying cancer metastasis and chemotherapy resistance, with a central focus on the ovarian cancer stem cells, will shed light on novel therapeutic strategies that can improve clinical outcomes in patients with advanced ovarian cancer.
2. Identification and Characterization of the Ovarian Cancer Stem Cell Population
The clinical course of ovarian cancer treatment typically results in a vast majority of patients undergoing relapse with chemoresistant metastatic tumors despite high response rates to first-line chemotherapy. In this regard, even though ovarian cancer stem cells have not been completely elucidated [
20,
21,
22], a small population of metastasized cancer cells with a high degree of chemoresistance may possess cancer stem cell properties and play a crucial role in relapse at metastatic sites [
23,
24,
25]. Therefore, elucidating the biology of ovarian cancer stem cells will yield novel insights into the still elusive mechanisms of metastasis and chemotherapy resistance in ovarian cancer.
In recent years, various specific markers of stemness, including CD44, CD117, aldehyde dehydrogenase (ALDH), CD133, CD24, and the epithelial cell adhesion molecule (EpCAM), have been used to isolate and characterize ovarian cancer stem cells [
26,
27,
28,
29,
30,
31,
32] (
Table 1). Zhang et al. identified a self-renewing subpopulation of ovarian cancer cells that can serially propagate their original tumor phenotype in an in vivo mouse model [
33]. Additionally, the authors found that sphere-forming cells co-express CD44 and CD117 and that those two specific markers can be used to isolate highly tumorigenic ovarian cancer stem cells [
33]. Similarly, Silva et al. demonstrated that ALDH and CD133 expression can be used to define distinct heterogeneous subpopulations of ovarian cancer stem cells. By using an in vivo mouse model, they showed that ALDH enzymatic activity and CD133 positivity were closely correlated with ovarian cancer stem cell phenotypes with high tumorigenic potential [
34]. In addition, Gao et al. isolated a series of cancer cell clones from ovarian cancer specimens and identified a subpopulation enriched for ovarian cancer stem cells defined by CD24 expression. The authors also found that CD24-positive ovarian cancer cells preferentially express higher levels of stem cell genes—including Nestin, β-catenin, Bmi-1, Oct4, Oct3/4, Notch1, and Notch4—than the CD24-negative counterpart, and they contribute to a specific capacity for self-renewal and multi-differentiation [
35]. In a related move, our research group previously generated mouse ovarian tumor-initiating cells by the siRNA-mediated knockdown of tumor suppressor p53 followed by the transduction of c-Myc and K-Ras oncogenes [
36]. Intriguingly, we also identified a subpopulation of EpCAM-positive cancer cells as possible candidates for ovarian cancer stem cells in an established mouse ovarian cancer model. By using an in vivo limiting dilution assay, we found that EpCAM-positive cancer cells isolated from hierarchically organized ovarian tumors have greater tumor-initiating properties than EpCAM-negative cancer cells. Additionally, with respect to the differentiation capacity of EpCAM-positive cancer cells, we showed that these cells can give rise to less tumorigenic EpCAM-negative cancer cells, attesting to the multi-lineage differentiation potential of these cells [
36].
Table 1. Ovarian cancer stem cell markers and stem cell signaling pathways.
| Marker/Signaling Pathway |
Type of Protein |
Function in Ovarian Cancer Stem Cell |
Reference |
| CD44 (CD44v6) |
Glycosylated transmembrane receptor |
Tumor initiation, transcoelomic metastasis, hematogenous metastasis, chemoresistance |
[27,30,33,37,38,39,40] |
| CD117 |
Tyrosine kinase receptor |
Tumor initiation, transcoelomic metastasis, chemoresistance |
[33,41,42] |
| ALDH |
Enzyme responsible for oxidizing intracellular aldehydes |
Tumor initiation, transcoelomic metastasis, hematogenous metastasis, chemoresistance |
[29,31,34,40,41,43,44,45] |
| CD133 |
Pentaspan transmembrane glycoprotein |
Tumor initiation, adhesion, invasion, transcoelomic metastasis, chemoresistance |
[28,29,32,34,40,46] |
| CD24 |
Mucin-like cell surface glycoprotein |
Tumor initiation, self-renewal, multi-differentiation, transcoelomic metastasis, chemoresistance |
[27,35,45,47] |
| EpCAM |
Type I transmembrane glycoprotein |
Tumor initiation, multi-differentiation, sphere formation, chemoresistance, prevention of chemotherapy induced-apoptosis |
[27,36,45,48] |
| Wnt |
Palmitoylated secreted glycoprotein |
chemoresistance |
[42,45,49] |
| Nothch |
Type I transmembrane glycoprotein |
chemoresistance |
[50,51] |
| Hedgehog |
Secreted signaling protein |
chemoresistance |
[52,53,54] |
Taken together, it appears that there are currently a significant number of markers that can be used to isolate a specific ovarian cancer stem cell population; however, these markers have not proven to be ubiquitously expressed in a given tumor, and it remains challenging to identify bona fide ovarian cancer stem cells. In view of this, further studies are needed to consistently enrich the subpopulation of ovarian cancer stem cells and to precisely explain the biology of ovarian cancer stem cells (Table 1).
3. High Metastatic Potential of Ovarian Cancer Stem Cells
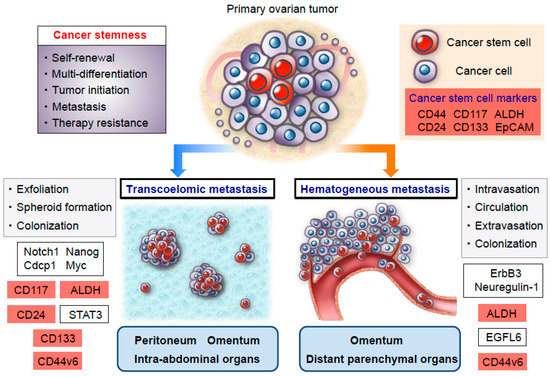
Ovarian cancer has been generally considered to preferentially metastasize via transcoelomic rather than hematogenous dissemination [
55,
56,
57]. Transcoelomic metastasis is implicated in the exfoliation of cancer cells from primary ovarian tumors, their survival as multicellular spheroids that float in the ascitic fluid, and their subsequent metastatic colonization in the peritoneal cavity [
55,
58,
59,
60] (
Figure 1). Previous studies demonstrated that ovarian cancer stem cells play a pivotal role in the formation of multicellular spheroids during the transcoelomic peritoneal dissemination of ovarian cancer [
61,
62]. Liao et al. showed that spheroids in the malignant ascites are enriched in cancer stem cells that are primarily responsible for ovarian cancer tumorigenesis, progression, and transcoelomic metastasis [
41]. The authors also revealed that these sphere-forming cancer cells express stemness-related genes—such as Notch1, Nanog, Cdcp1, and Myc—and upregulate CD117 expression and ALDH activity, thereby promoting the development of peritoneal and omental metastasis in an in vivo ovarian cancer xenograft model [
41]. More recently, Roy et al. elucidated the functional role of CD133 expression in the early steps of peritoneal metastasis. Using an ex vivo peritoneal explant adhesion assay, they demonstrated that CD133 expression significantly increased the adhesion and invasion of ovarian cancer cells to the peritoneal mesothelium, suggesting that CD133-positive ovarian cancer cells are involved in communicating with the microenvironmental niche in the peritoneum [
46]. In this regard, a previous study identified a key role for peritoneal mesothelial cells in the maintenance of ovarian cancer stemness, thus raising the possibility that the peritoneal microenvironment has the potential to form a cancer stem cell niche for ovarian cancer dissemination [
63]. In addition, a recent study by Burgos-Ojeda et al. showed that CD24-positive ovarian cancer cells manifest increased levels of STAT3 phosphorylation compared with CD24-negative cells, an increase which is associated with enhancing disseminated metastasis [
47]. More importantly, the blockade of STAT3 phosphorylation with a JAK 2 inhibitor significantly reduced ovarian cancer metastasis in an established genetic mouse model of ovarian cancer [
47]. On another front, our study group demonstrated a crucial role for CD44 variant 6 (CD44v6) in the regulation of various biological functions during the transcoelomic metastasis of ovarian cancer [
37]. A clinicopathological analysis of CD44v6 expression indicated that peritoneal disseminated tumors are highly enriched in CD44v6-positive ovarian cancer cells compared with corresponding primary tumors, suggesting that CD44v6-positive cancer cells are associated with peritoneal transcoelomic dissemination and that the peritoneum may function as a metastatic microenvironmental niche that contributes to the process of metastatic colonization in the peritoneal cavity. Intriguingly, and consistent with these clinical observations, a subpopulation of CD44v6-positive cancer cells gave rise to extensive disseminated metastatic tumors in the peritoneal cavity, whereas CD44v6-negative cancer cells manifested a lower metastatic ability in an in vivo mouse model. Furthermore, a limiting dilution assay demonstrated that CD44v6-positive cancer cells showed a greater tumor initiating capability than CD44v6-negatice cancer cells, indicating that the subpopulation of CD44v6-positive cancer cells may serve as specialized metastasis-initiating cells within the intraperitoneal milieu [
37] (
Figure 1 and
Table 1).
Figure 1. Schema of an ovarian cancer stem cell model for transcoelomic and hematogenous metastasis. A subpopulation of ovarian cancer stem cells has greater tumor-initiating properties and serves as functionally and molecularly distinct metastasis-initiating cells. Especially, ovarian cancer stem cells are responsible for not only driving transcoelomic dissemination but also hematogenous metastasis via the activation of various signaling pathways involved in stem cell biology. Cancer stem cell markers are highlighted in red.
Generally, although transcoelomic dissemination has been thought to be the major route of ovarian cancer metastasis [
60], a recent study by Pradeep et al. revealed novel mechanisms of hematogenous metastasis to the omentum followed by intraperitoneal disseminated metastasis in a parabiosis mouse model that allowed for the sharing of blood circulation [
64]. They showed that circulating ovarian cancer cells derived from the host mouse can first metastasize to the omentum of the conjoined guest mouse via a hematogenous route and subsequently spread to the peritoneal cavity, thus shifting the paradigm in mechanisms that regulate the disseminated metastasis of ovarian cancer in the peritoneal cavity. Mechanistically, the ErbB3/Neuregulin-1 signaling axis has been shown to play a functional role in such hematogenous ovarian cancer metastasis with a strong tropism toward the omentum [
64]. Additionally, with respect to distant parenchymal metastasis via the hematogenous route in ovarian cancer, our recent study demonstrated that CD44v6-positive ovarian cancer cells represent a central player in the development of distant metastasis in parenchymal organs [
38] (
Figure 1). Clinicopathological evidence corroborated the fact that ovarian cancers with greater numbers of CD44v6-positive cancer cells were associated with higher rates of distant metastasis at the time of ovarian cancer diagnosis. Furthermore, an immunohistochemical analysis detected a significantly higher percentage of CD44v6-positive cells in distant metastatic tumors than primary ovarian tumors, suggesting that CD44v6-positive cancer cells play a key role in driving distant metastasis via a hematogenous spread. More importantly, a Kaplan–Meier analysis showed that distant metastasis-free survival was significantly different between CD44v6-high and -low groups, indicating that CD44v6 expression is involved in greater distant metastatic relapse in patients with stage I–III ovarian cancer. It should be noted here that a multivariate analysis identified CD44v6 expression as an independent risk factor for distant metastatic relapse, implying that CD44v6 expression may be a crucial predictive biomarker for distant parenchymal metastasis in ovarian cancer patients [
38]. A number of studies of late years have indicated that ALDH activity is closely linked to both ovarian cancer stemness and metastasis [
65,
66]. Bai et al. showed that epidermal growth factor-like domain 6 (EGFL6), which acts as a stem cell regulatory factor, promotes the asymmetric division of ALDH-positive ovarian cancer stem cells and thereby increases cancer cell proliferation in vitro and tumor growth in vivo [
43]. Interestingly, the authors also found that vascular EGFL6 expression enhances the distant metastasis of ovarian cancer cells through the hematogenous route, whereas an EGFL6 blockade reduces the hematogenous spread of ovarian cancer cells to distant parenchymal organs [
43]. These findings provide a therapeutic rationale for targeting specific molecules in the microenvironmental niche (
Figure 1 and
Table 1).
This entry is adapted from the peer-reviewed paper 10.3390/cancers11070907