Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Genetics & Heredity
The introduction of herbaceous peony (Paeonia lactiflora Pall.) in low-latitude areas is of great significance to expand the landscape application of this world-famous ornamental. With the hazards of climate warming, warm winters occurs frequently, which makes many excellent northern herbaceous peony cultivars unable to meet their chilling requirements (CR) and leads to their poor growth and flowering in southern China. Exploring the endodormancy release mechanism of underground buds is crucial for improving low-CR cultivar screening and breeding.
- abscisic acid
- chilling requirement (CR)
- endodormancy release
- herbaceous peony
- Paeonia lactiflora Pall
- reactive oxygen species
- underground bud dormancy
1. Introduction
Bud dormancy is an important survival strategy of temperate perennial plants in response to detrimental environments, such as cold in winter. With global climate change, bud dormancy has gradually become a popular topic in plant growth and development research fields in recent decades [1,2]. The complete process of bud dormancy includes three contiguous stages: paradormancy, endodormancy and ecodormancy [3]. Paradormancy is well accepted as the cessation of bud growth after shoot elongation stops. As the daylength shortens and the temperature decreases, perennials defoliate, and their buds generally transfer from paradormancy to endodormancy [4]. In autumn, with a decrease in temperature or day length, buds enter endodormancy, at which stage they are unable to sprout even under favorable conditions until the indispensable chilling accumulation has been completed [5,6]. Ecodormancy is the dormancy caused by adverse environmental factors such as low temperature and drought stress [3]. Once the environmental conditions are suitable, dormancy is released. However, endodormancy cannot be released before accomplishing indispensable chilling accumulation. Due to the global warming trend, many plants with winter dormancy traits suffer from hot autumns and warm winters, which disrupt normal endodormancy release because of insufficient chilling accumulation and then negatively affect their subsequent growth and development. Sufficient daylength and low temperature are the two most important environmental factors affecting the bud dormancy process. Short daylength could induce Populus L. and Morus alba L. to enter dormancy, and a low-temperature environment could induce Pyrus spp. and Malus pumila Mill. to enter dormancy [7,8,9]. Shortening daylength alone does not induce these fruit trees to enter bud dormancy without decreasing temperature. The dormancy of Vitis vinifera L. and Armeniaca vulgaris Lam. was mainly controlled by sunshine duration and low temperature [10,11].
The internal factors controlling dormancy processes are complicated and involve carbohydrate conversion, hormone signaling, antioxidant metabolism, water transport, gene regulation, and DNA methylation and demethylation [12,13,14]. During the paradormancy stage, sugar metabolic activity is continuously vigorous, and the large amount of accumulated soluble total sugar and sucrose not only helps to improve cell osmotic potential to resist the cold in winter but also acts as a regulatory substance to inhibit the growth of buds in perennials [15,16,17]. In addition, the content and balance of endogenous hormones are currently widely accepted to control the occurrence and release of bud endodormancy. A previous study showed that the formation and maintenance of abscisic acid (ABA) and the status transition of bud dormancy were closely interrelated in potato. The ABA content increased with the deepening of bud endodormancy but decreased regularly with the endodormancy release [18]. Generally, the ABA content continuously increased in paradormancy, reached the highest level in endodormancy and finally decreased to the lowest level in ecodormancy [18,19,20]. Gibberellin (GA) was confirmed to promote the dissolution of dormancy. The accumulation of low temperature in winter resulted in an increase in GA content and then promoted the degradation of callose in the sieve tube, which was conducive to promoting the release of endodormancy [21]. Additionally, reactive oxygen species (ROS) function as signal transduction factors and are also involved in many regulatory processes related to plant growth and development. The activities of superoxide dismutase (SOD) and peroxidase (POD) gradually increased, while catalase (CAT) activity showed the opposite trend after the end of endodormancy in pear [22].
In addition to the control of physiological factors, bud endodormancy induction, transition and release are also regulated by various genes and associated pathways. Hormone signaling and metabolism-related genes, such as 9-cis-epoxycarotenoid dioxygenase (NCED), ABA-insensitive 5 (ABI5), protein phosphatase 2ca (PP2C), and Arabidopsis thaliana gibberellin 2-oxidase (GA2ox), are popular research topics in plant bud endodormancy, while sucrose synthase 3 (SUS3), Arabidopsis thaliana cell wall invertase 1 (CWINV), cyclin D3 (CYCD) and cyclin-dependent kinase b2;2 (CDKB22) are also frequently involved in carbohydrate and ROS metabolism. These genes promote or inhibit plant bud endodormancy and may play an important role in breeding plant germplasm with long or short endodormancy periods through gene overexpression and silencing. In addition, dormancy-associated MADS-box (DAM) genes belong to the MADS-box gene family and are crucial for bud endodormancy regulation [23]. DAM genes were isolated and identified in a study on bud endodormancy in peach with rare evergreen traits [24]. Since then, researchers have identified related genes in many other species (pear, plum, kiwifruit) and further confirmed their association with the endodormancy process [25,26,27]. These genes form a complex dormancy regulatory network with other MADS-box genes and genes related to carbohydrate, phytohormone and antioxidant metabolism [28].
The bud endodormancy of Paeonia suffruticosa belonging to the same genus as Paeonia lactiflora has been studied for decades. During the process of herbaceous peony flower bud endodormancy release, accompanied by the hydrolysis of soluble sugar, starch, and the increase of antioxidant enzyme activity [29]. Hormone regulation of herbaceous peony flower bud endodormancy is mainly dependent on the ratio of endodormancy release promoter to inhibitor, especially GA and ABA, the ratio between the two is closely related to the endodormancy process of herbaceous peony flower bud [18,30,31]. In addition, in-depth research at the molecular level has mainly focused on methylation and gene regulation [32,33]. GA and low temperature both have the effect of reducing DNA methylation. The gene GA2ox8 related to GA synthesis is also one of the important genes regulating endodormancy [34].
Herbaceous peony (Paeonia lactiflora Pall.) is a world-famous ornamental plant that originates from China and has a long cultivation history worldwide [35]. It is widely used in cut flowers, potted plants and gardens. This species is mainly distributed in temperate and cool regions of the Northern Hemisphere, such as Europe, North America and Asia [36]. To survive in cold winters, the aboveground parts of herbaceous peony wither in autumn, and the underground bud undergoes endodormancy. Only after accumulating enough of a chilling requirement (CR) in winter can it grow well in the coming year. Therefore, chilling is an essential factor in the endodormancy-growth cycle of herbaceous peony [37]. In this dormant period, the underground buds of herbaceous peony undergo a series of physiological changes, such as carbohydrate conversion, hormone metabolism and ROS signal transduction [38]. The introduction of herbaceous peony from high latitudes to low latitudes is beneficial to expand its distribution range and increase its application in landscaping. However, the winter temperature at low latitudes is too high to meet the CR, which poses a great obstacle to the release of bud endodormancy in the coming spring [39,40].
3. Discussion
3.1. CR Evaluation Is an Important Method to Popularize Herbaceous Peony at Low Latitudes
Compared with long-term breeding, CR evaluation and screening of cultivars with low cold demand is more direct and can help select target cultivars in a shorter time. Typical materials with high cold demand and low cold demand can be selected after evaluation and can be used as parents for scientific research and long-term breeding [44]. For herbaceous peonies, it will take a long time to create new cultivars with the low-CR trait by a traditional crossbreeding strategy. Therefore, the introduction of herbaceous peonies from high-latitude areas and CR evaluation are needed to screen for low-CR cultivars [37]. In this study, the CR of ‘Meiju’ was calculated as 677.5 CUs by the UT model, and thus ‘Meiju’ was verified as a low-CR cultivar compared with other northern cultivars (‘Zhuguang’: 1182 CUs, ‘Qiaoling’: 1017 CUs, ‘Qihua Lushuang’: 685.5 CUs, ‘Fen Yunu’: 685.5 CUs [37]). In our previous study, the bud endodormancy of ‘Meiju’ was released on 9 January 2017 and the CR was correspondingly calculated as 1017.0 CUs based on the UT model [37]. As a matter of fact, we analyzed the morphological data more carefully afterwards, and deduced that the bud endodormancy of ‘Meiju’ had probably been released on 26 December 2016. Therefore, the true CR value of ‘Meiju’ should be 685.5 CUs during the winter of 2016 to 2017, which is very close to 677.5 CUs verified in this study. This northern cultivar grew vigorously and bloomed exuberantly in Hangzhou based on multiyear observations; thus, it could be used as a pioneer cultivar to popularize herbaceous peony in Hangzhou and other low-latitude areas [37,41].
3.2. Carbohydrate Metabolism Participates in the Regulation of Endodormancy Release
The conversion of sucrose, total soluble sugar and starch is an important factor in regulating bud endodormancy in perennials [15,16,17,45,46]. Generally, in the stage of endodormancy, starch tends to decrease, while sucrose and total soluble sugar increase accordingly. Starch is a polysaccharide that cannot be directly used by plants, and it can only be used when it is converted into monosaccharides. Therefore, the increase in sucrose and total soluble sugar content in herbaceous peony buds before 9 January may come from the decomposition of starch, which can provide energy for the life activities of herbaceous peony buds and promote the gradual release of endodormancy in herbaceous peony buds [15,16]. However, this is different from the results from a study in spinach, which shows that different species have different regulatory mechanisms [47].
On the other hand, the increase in sucrose and total soluble sugar content may also help to reduce the osmotic pressure of cells and ensure that the cells can fully absorb water, thus increasing the cold resistance of herbaceous peony buds and helping them survive the lowest temperature period in Hangzhou in January (Figure 2) [48].
3.3. Relationship between Hormone Metabolism and Endodormancy Release
Plant endogenous hormones play an important role in the induction, transformation and release of bud endodormancy [4,49,50]. Plant endogenous hormones mainly include two categories: promoting the endodormancy release of buds (such as GA, ZR, BR, JA, and IAA) and inhibiting the release (maintaining endodormancy, such as ABA) [12,51,52]. The endogenous hormone content in plants is an important factor regulating the induction and release of endodormancy, and the dynamic balance between hormone contents is often highly correlated with the process of dormancy [4,50].
Figure 10 shows that among the genes associated with ABA, only the expression pattern of ABI5 was completely consistent with the change in ABA level from endodormancy to ecological dormancy. ABI5 is a key transcription factor in the ABA signaling pathway that helps to promote ABA signal transduction [44]. The rapid downregulation of ABI5 around 9 January may be one of the reasons for the decrease in ABA content, which is the key hormone-related gene to accelerate the release of endodormancy [53].
As an ABA binding factor, ABF2 often combines with AREB1 to form the AREB1/ABF2 transcription factor, which is the key factor downstream of the ABA signaling pathway [54,55]. In the process of bud dormancy, the expression of ABF2 is theoretically conducive to ABA signal transduction, thus maintaining bud endodormancy [6,56]. In this study, ABF2 showed a unimodal curve, which reached its peak on 9 January (Figure 10), suggesting that its high expression promoted the release of endodormancy in buds. However, the trend of ABF2 expression during the whole endodormancy period was opposite that of ABA (Figure 6 and Figure 10), suggesting that its expression was upregulated but that the ABA content was decreased.
However, the expression of other genes is only in line with expectations at some stages. For example, the expression of upstream genes NCED3 and NCED4 in the ABA synthesis pathway increases during endodormancy (Figure 10, 17 October–9 January); this gene has been proven to be an important gene for promoting ABA synthesis, which is consistent with the results of ABA content determination (Figure 6) [31,57,58]. One of the possible reasons why ‘Meiju’ is a low CR herbaceous peony is that the NCED gene is upregulated in early winter. However, in the stage of ecodormancy in which the content of ABA decreased, NCED3 and NCED4 continued to be upregulated and maintained at a relatively high expression level (9 January–27 February). Similar results are also reflected in pear, which shows that the genes in the NCED gene family regulate different biological processes, and these genes may also be involved in the resistance of herbaceous peony buds to low temperature in winter during ecodormancy [4].
GA3 is one of the important hormones regulating bud endodormancy [59]. In this study, the content of GA3 was gradually decreased before the endodormancy release, and the decrease might be related to the antagonism of ABA. In the stage when endodormancy release (before 9 January), the ABA content decreased rapidly and the ratio of GA/ABA began to increase, which promoted the release of endodormancy. In terms of gene expression, GA2ox has been proved to have a negative regulatory effect on GA synthesis in Arabidopsis thaliana and poplar [60,61]. After the endodormancy released, the rapid increase in GA3 content might be related to the up-regulation of GASA4 gene expression, which is related to gibberellin synthesis.
In summary, the chilling requirement of ‘Meiju’ is shorter, and the endodormancy release period is earlier, which is closely related to the early decrease in ABA content and the regulation of related genes such as ABI5, NCED3, and PP2C [42,58,62,63]. More gene function verification experiments are needed to confirm this hypothesis. In addition to ABA, hormones that promote the release of bud endodormancy, such as GA3, IAA, JA, ZR and BR, which act in opposition to ABA, can also be involved in determining the characteristics of low chilling requirements [64,65,66,67].
3.4. Antioxidant Metabolism Involved in Endodormancy Release Regulation
Many studies have shown that antioxidant metabolism is also involved in the regulation of bud endodormancy [22,68]. Similar to a study in pears, the activity of SOD decreased before the release of ecodormancy but began to increase after this release, and the activity of CAT gradually increased before the termination of endodormancy and began to decline after termination [22]. The activity of POD was upregulated rapidly before the release of endodormancy and then decreased rapidly. These findings are completely different from those in pears [22]. The enzyme activity related to the antioxidant metabolic pathway responded rapidly during paradormancy and coincided with the degree of dormancy. This suggests that ROS may act as a signal transduction substance to sense environmental changes and participate in the regulation of paradormancy induction and release. However, the related regulatory mechanisms may be different in different species [22,69].
At the level of gene expression, the expression of most genes related to antioxidant metabolism (EXPA6, CDKB22, CYCD3, BG3, actin-related protein 6 (ARP6), PER52, histone h1-3 (HIS1-3)) decreased gradually before the release of endodormancy and then increased again (Figure 9). The expression of these genes changed dramatically, decreased rapidly when entering endodormancy, and then increased rapidly after endodormancy was released.
In other metabolic pathways, the expression trend of genes related to ROS signal transduction was opposite those related to sucrose content (Figure 5), total soluble sugar content (Figure 5) and ABA content (Figure 6). ROS may play a role in sensing and transmitting signals in the process of dormancy release in buds of ‘Meiju’. This shows that the efficient signal transmission of ROS and the timely reflection of other physiological activities may be one of the reasons for the low cooling requirement of ‘Meiju’.
3.5. Judgment of Endodormancy Release Should Be Based on Comprehensive Research
According to the experiment of moving outdoor potted herbaceous peony to a greenhouse, several key indices (BPF, ANS, APW, APH, ADS, WFS) were used as the most intuitive evidence to judge that the endodormancy of herbaceous peony was released around 9 January.
Additionally, the state of cells in leaf primordia (LP), growing point (GP), vascular strand primordia (VS), bud primordia (BP) and other parts is also quite different from that in the previous period, with an obvious tight arrangement of cells and a deeper color of nuclei (Figure 4). The continuous accumulation of these soluble carbohydrates possibly helped to increase the osmotic potential of underground bud cells and enhanced the cold resistance of ‘Meiju’ (Figure 5). High concentrations of sugar, together with ROS signaling, may lead to a decrease in the contents of hormones such as ABA, JAME, and IAA (Figure 12). The expression of many important genes related to endodormancy release, such as CBF4, PP2C, and NCEDs, reached the highest level around 9 January (Figure 10) [41,47,48,50].
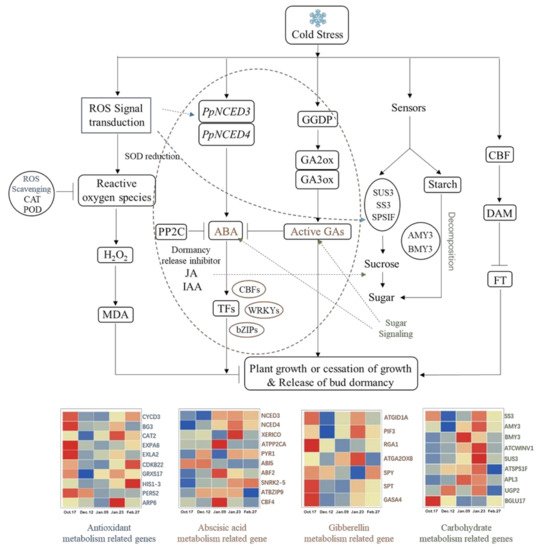
Figure 12. Underground bud endodormancy regulatory network. The picture was drawn by TBtools and Microsoft Office 2019. The data were processed by IBM SPSS Statistics 26. Full name and function of the genes are shown in Table 2.
These indices may be used as potential judgment indices of CR satisfaction and the endodormancy release period to provide some new judgment indices for the general method of endodormancy release when the germination rate reaches 50%, but they need to be further studied and verified [70].
3.6. Possible Regulatory Networks for Dormancy Release of Underground Buds in Herbaceous Peony
Temperature is closely related to the induction, maintenance and release of perennial bud endodormancy [45,71]. It has been proven that there is a relationship between cold signal transduction and molecular networks related to bud endodormancy in some temperature-sensitive plants [72]. There is a great similarity between these plants and ‘Meiju’. In the underground bud of ‘Meiju’, the change in external temperature causes ‘Meiju’ to produce a large amount of MDA, causing damage to the bud of “Meiju”.ROS signal transduction responded quickly and transmitted signals to other related metabolic processes in time, among which glucose metabolism and hormone metabolism are the most important. The ABA content increased rapidly through the regulation of the NCED3 and NCED4 genes. In the later stage of endodormancy, GA2ox8 and other genes regulated gibberellin accumulation and inhibited the effect of ABA. Under the joint regulation of the DAM gene, the endodormancy of ‘Meiju’ underground buds was released (Figure 12).
A large number of antioxidant metabolism-related genes and some carbohydrate metabolism-related genes (SS3, BGLU) responded quickly (Figure 12). Which made the sugar and hormone metabolism extremely active, allowing earlier completion of the accumulation of soluble sugars and the synthesis and metabolism of endodormancy release inhibitor (ABA). Therefore, the ABA/GA ratio decreased rapidly in the earlier period (9 January), which may be an important reason for the short endodormancy period and low cooling capacity of ‘Meiju’.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168382
This entry is offline, you can click here to edit this entry!
