Poor oral hygiene is the primary cause of common oral diseases. Accumulation of dental plaque allows bacterial growth that may lead to inflamed periodontal tissues and eventually create bacteremia and systemic inflammation. Invading bacteria from severe caries or endodontic infections is also thought to provoke similar mechanisms.
- oral hygiene
- dental plaque
- oral bacteria
- tooth brushing
- interdental cleaning
- dental visit
- metabolic syndrome
1. Introduction
Metabolic syndrome (MetS), a clustering of abdominal obesity, hyperglycemia, hypertension, and dyslipidemia, represents a growing public health concern globally [1]. Although the prevalence of MetS differs depending on diagnostic criteria, age group, and ethnicity [1][2], it is estimated to affect around 25% of the world population [2][3]. MetS raises the risk of type 2 diabetes mellitus (T2DM) and cardiovascular diseases [1] and is associated with a 20% increase in healthcare costs [4].
Several risk factors for MetS have been identified. Besides socioeconomic status (SES) [5], smoking [6], diet [7], and physical activity [8], oral diseases, such as periodontal diseases and dental caries, are associated with MetS [9][10][11]. The link between oral and systemic diseases is suggested due to common risk factors, subgingival biofilm harboring Gram-negative bacteria, and periodontium serving as a cytokine reservoir [12].
Tooth brushing and interdental cleaning, which are the main forms of oral self-care, together with regular professional care, are important measures for plaque control or removal and maintaining optimal oral health [13][14][15]. Poor oral hygiene care is associated with low-grade inflammation [16], suggesting its potential link to MetS [17]. The association of poor oral hygiene care with a higher risk of the components of MetS, such as obesity [18], diabetes [19][20], hypertension [20][21], and dyslipidemia [20][22], as well as with cardiovascular disease [23][16], has been demonstrated.
Although several epidemiological studies have reported the association of oral hygiene status [24] and care [17][25] with MetS, some studies found no such association [26][27]. To date, there has not been a systematic review conducted on the topic. A summary of evidence can provide a better understanding of the potential relationship and help healthcare practitioners deliver more targeted care. It can provide more substance for the formulation of public health programs and policies, especially strategies for the prevention and management of MetS.
2. Association between Oral Hygiene Status, Care, and MetS
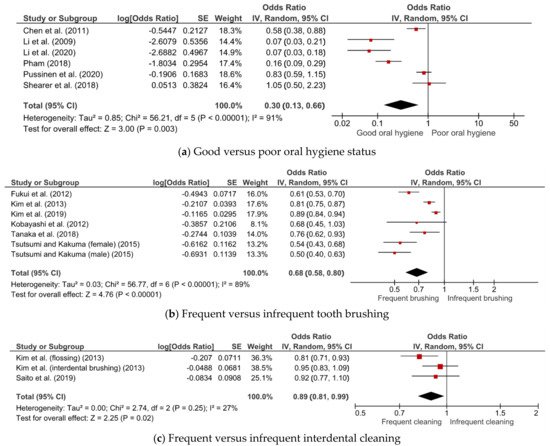
3. Subgroup Analyses
| Subgroup | Number of Studies | OR (95% CI) | I2 (%) | p |
|---|---|---|---|---|
| Cross-sectional | 2 | 0.72 (0.41–1.26) | 46 | 0.17 |
| Case–control | 3 | 0.11 (0.06–0.20) | 39 | 0.19 |
| Cohort | 1 | 0.83 (0.59–1.15) | - | - |
| Subgroup | Number of Studies | OR (95% CI) | I2 (%) | p |
|---|---|---|---|---|
| Study design | ||||
| Cross-sectional | 5 | 0.67 (0.55–0.81) | 93 | <0.001 |
| Cohort | 2 | 0.74 (0.62–0.89) | 0 | 0.64 |
| Country | ||||
| Japan | 5 | 0.61 (0.52–0.70) | 55 | 0.06 |
| Korea | 2 | 0.85 (0.78–0.93) | 73 | 0.06 |
4. Conclusion
This entry is adapted from the peer-reviewed paper 10.3390/jcm10132873
References
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822.
- Lear, S.A.; Gasevic, D. Ethnicity and metabolic syndrome: Implications for assessment, management and prevention. Nutrients 2020, 12, 15.
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8.
- Curtis, L.H.; Hammill, B.G.; Bethel, M.A.; Anstrom, K.J.; Gottdiener, J.S.; Schulman, K.A. Costs of the metabolic syndrome in elderly individuals: Findings from the Cardiovascular Health Study. Diabetes Care 2007, 30, 2553–2558.
- Blanquet, M.; Legrand, A.; Pélissier, A.; Mourgues, C. Socio-economics status and metabolic syndrome: A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1805–1812.
- Sun, K.; Liu, J.; Ning, G. Active Smoking and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. PLoS ONE 2012, 7, e47791.
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary patterns and metabolic syndrome in adult subjects: A systematic review and meta-analysis. Nutrients 2019, 11, 2056.
- Joseph, M.S.; Tincopa, M.A.; Walden, P.; Jackson, E.; Conte, M.L.; Rubenfire, M. The impact of structured exercise programs on metabolic syndrome and its components: A systematic review. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2395–2404.
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 1035–1057.
- Cao, X.; Wang, D.; Zhou, J.; Yuan, H.; Chen, Z. Relationship between dental caries and metabolic syndrome among 13 998 middle-aged urban Chinese. J. Diabetes 2017, 9, 378–385.
- Ojima, M.; Amano, A.; Kurata, S. Relationship between decayed teeth and metabolic syndrome: Data from 4716 middle-aged male Japanese employees. J. Epidemiol. 2015, 25, 204–211.
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558.
- Claydon, N.C. Current concepts in toothbrushing and interdental cleaning. Periodontol. 2000 2008, 48, 10–22.
- Ainamo, J. Prevention of periodontal disease in the dental office. Int. Dent. J. 1984, 34, 56–61.
- Lim, L.P.; Davies, W.I.R. Comparison of various modalities of “simple” periodontal therapy on oral cleanliness and bleeding. J. Clin. Periodontol. 1996, 23, 595–600.
- De Oliveira, C.; Watt, R.; Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: Results from Scottish Health Survey. BMJ 2010, 340, 1400.
- Tanaka, A.; Takeuchi, K.; Furuta, M.; Takeshita, T.; Suma, S.; Shinagawa, T.; Shimazaki, Y.; Yamashita, Y. Relationship of toothbrushing to metabolic syndrome in middle-aged adults. J. Clin. Periodontol. 2018, 45, 538–547.
- Nijakowski, K.; Lehmann, A.; Rutkowski, R.; Korybalska, K.; Witowski, J.; Surdacka, A. Poor oral hygiene and high levels of inflammatory cytokines in saliva predict the risk of overweight and obesity. Int. J. Environ. Res. Public Health 2020, 17, 6310.
- Chang, Y.; Lee, J.S.; Lee, K.J.; Woo, H.G.; Song, T.J. Improved oral hygiene is associated with decreased risk of new-onset diabetes: A nationwide population-based cohort study. Diabetologia 2020, 63, 924–933.
- Fujita, M.; Ueno, K.; Hata, A. Lower frequency of daily teeth brushing is related to high prevalence of cardiovascular risk factors. Exp. Biol. Med. 2009, 234, 387–394.
- Choi, H.M.; Han, K.; Park, Y.-G.; Park, J.-B. Associations Among Oral Hygiene Behavior and Hypertension Prevalence and Control: The 2008 to 2010 Korea National Health and Nutrition Examination Survey. J. Periodontol. 2015, 86, 866–873.
- Song, T.J.; Kim, J.W.; Kim, J. Oral health and changes in lipid profile: A nationwide cohort study. J. Clin. Periodontol. 2020, 47, 1437–1445.
- Chang, Y.; Woo, H.G.; Park, J.; Lee, J.S.; Song, T.J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study. Eur. J. Prev. Cardiol. 2020, 27, 1835–1845.
- Pham, T. The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int. J. Dent. Hyg. 2018, 16, 484–491.
- Kobayashi, Y.; Niu, K.; Guan, L.; Momma, H.; Guo, H.; Cui, Y.; Nagatomi, R. Oral health behavior and metabolic syndrome and its components in adults. J. Dent. Res. 2012, 91, 479–484.
- Pussinen, P.J.; Paju, S.; Viikari, J.; Salminen, A.; Taittonen, L.; Laitinen, T.; Burgner, D.; Kahonen, M.; Lehtimaki, T.; Hutri-Kahonen, N.; et al. Childhood Oral Infections Associate with Adulthood Metabolic Syndrome: A Longitudinal Cohort Study. J. Dent. Res. 2020, 99, 1165–1173.
- Shearer, D.M.; Thomson, W.M.; Cameron, C.M.; Ramrakha, S.; Wilson, G.; Wong, T.Y.; Williams, M.J.A.; McLean, R.; Theodore, R.; Poulton, R. Periodontitis and multiple markers of cardiometabolic risk in the fourth decade: A cohort study. Community Dent. Oral Epidemiol. 2018, 46, 615–623.
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28.
- Santarelli, A.; Wong, D.T.W.; Lo Muzio, L. Editorial: Saliva and Oral Microbiota: From Physiology to Diagnostic and Therapeutic Implications. Front. Physiol. 2021, 11, 637599.
- Souza, M.L.; Massignan, C.; Peres, K.G.; Peres, M.A. Association between metabolic syndrome and tooth loss: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 1027–1039.e7.
