In this study, we evaluated the neutralizing capacity in two groups of COVID-19 patients and healthcare professionals who had received the complete dose regimen of the BNT162b2 vaccine. For that purpose, an sVNT was used. The method was based on antibody-mediated blockage of the interaction between the ACE2 receptor protein and the RBD. Since some reports demonstrated that some non-RBD targeting antibodies could possess neutralizing capacity [
14,
15], the agreement of the sVNT with pVNT was evaluated using a subset of our cohort of COVID-19 patients. An excellent agreement of 97.2% was found and is consistent with the manufacturer’s data. We also found that the specificity of the sVNT using a panel of 250 pre-pandemic samples was excellent (i.e., 99.6%) using the manufacturer’s cut-off of 10.0 AU/mL. A potential cut-off refinement using a ROC curve analysis did not reveal the usefulness of an optimized cut-off, as already performed for some serological assays [
16,
17,
18,
19,
20,
21,
22]. The excellent specificity observed in our study was in line with that claimed by the manufacturer (i.e., 99.3%) using 270 samples from healthy volunteers who had no COVID-19 infection history and no vaccination history (manufacturer’s information). The precision of the assay was also good.
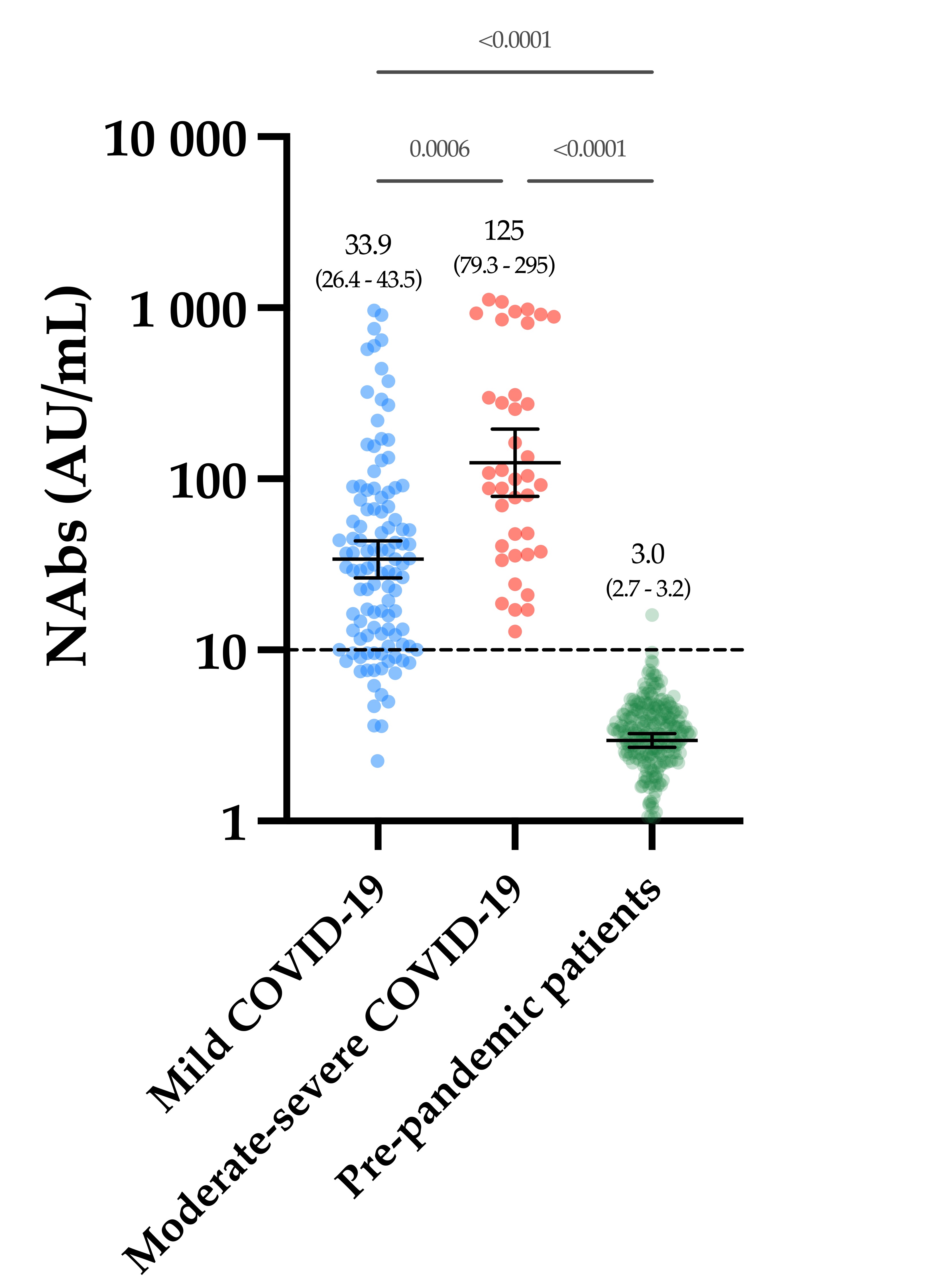
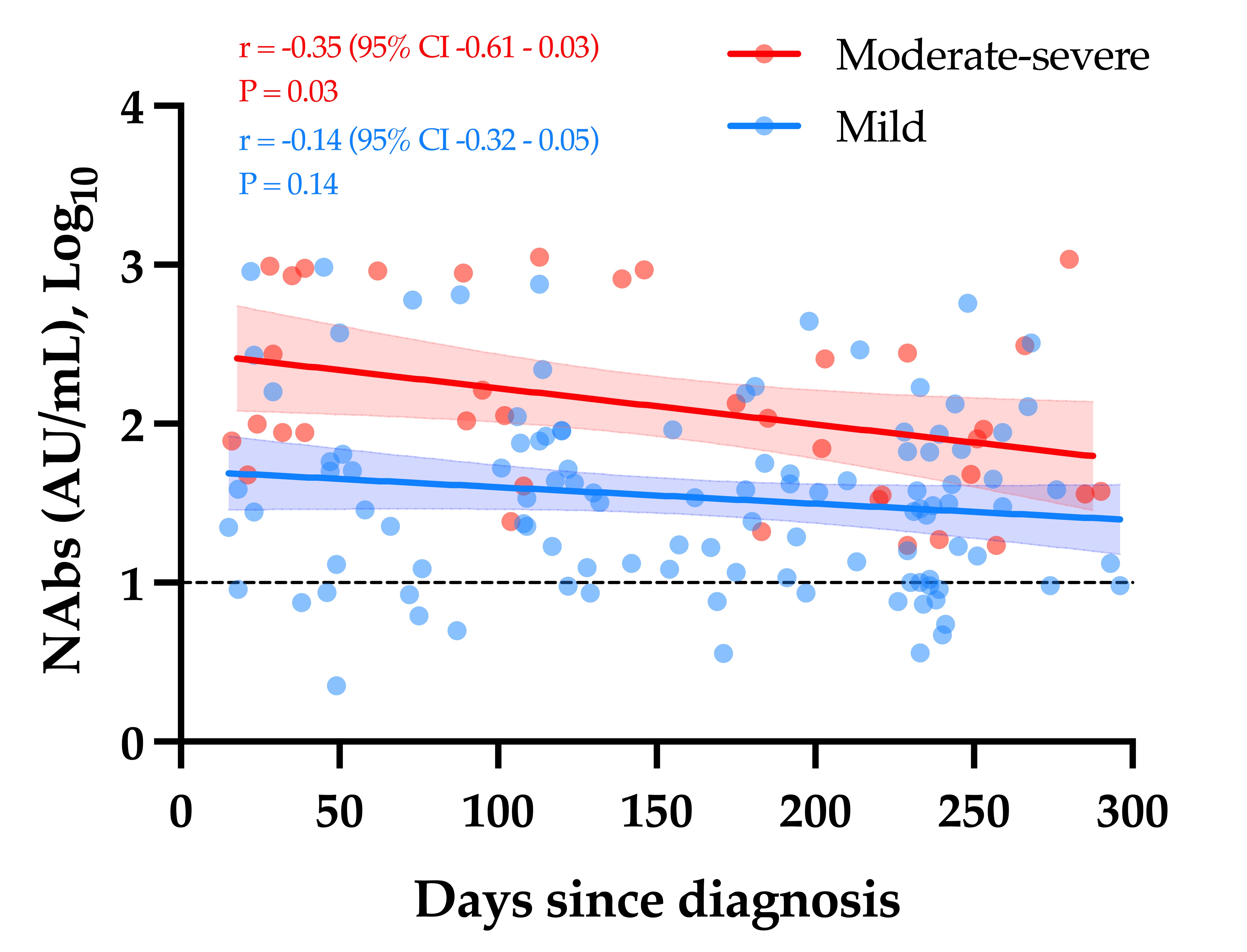
The slow decay in NAbs with time was also consistent with some reports [
23,
24,
25,
26,
27,
28,
29,
30], especially considering mild–moderate patients. A stronger SARS-CoV-2 antibody response in severe patients was also reported [
13]. Compared to SARS-CoV-2 antibody assays, only neutralization activity assays reliably measure the real protective immunity of generated antibodies. There is also a high demand for the neutralization tests in specific clinical and industrial settings (e.g., for identification purposes with convalescent plasma or to support the development of vaccines). However, the conventional virus neutralization test requires live pathogens and is reserved for very specialized laboratories, requiring a high workload, skillful operators, specific and expensive facilities, and a biosafety level 3 laboratory, and on top of that, they have a low result throughput [
8,
9]. The use of automated and quantitative assays with a short turn-around time that have a well-documented correlation with the neutralizing activity should be preferred [
7,
9,
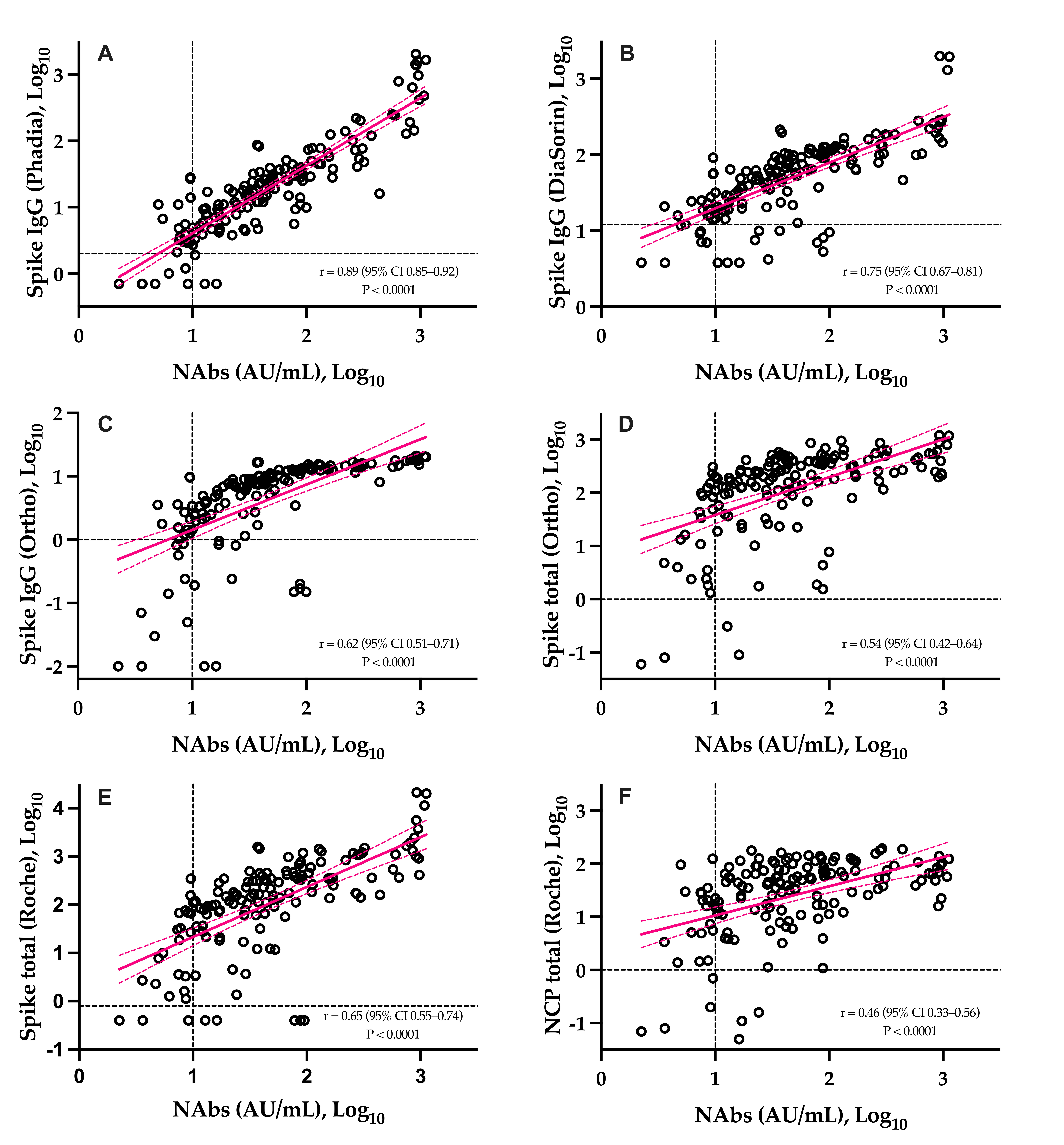
31]. In our study, we observed that the Phadia S1 IgG assay had the highest correlation compared to sVNT (r = 0.89) (
Figure 3). The second, better correlated assay was the DiaSorin S1/S2 assay (r = 0.75). This is in line with the findings of Legros et al., who showed a correlation of 0.71 using a microneutralization assay [
32]. The Ortho S1 IgG assay had a higher correlation compared to the Ortho S1 total assay, as observed in a study by Padoan et al. [
7]. Considering anti-NCP antibodies, the Roche total assay presented the lowest correlation with the results of the sVNT (r = 0.46). Patel et al. obtained similar conclusions when comparing the Roche NCP total assay to neutralizing activity (r = 0.40) [
33]. We therefore confirm that the strongest correlations are observed using anti-S or anti-RBD assays [
5,
29,
34,
35,
36] and our study highlights that correlations were especially high with the IgG assay. The fact that anti-NCP assays had a low correlation with the neutralizing activity was expected, as NAbs are directed against the S protein [
37]. Nevertheless, it is important to keep in mind that a few patients may develop specific antibodies, i.e., antibodies detected by conventional serological assays, which do not translate into a detectable neutralizing activity. We therefore think that the assessment of the neutralizing activity using an sVNT on an automated platform (without the disagreement of the gold standard technique) might be valuable.
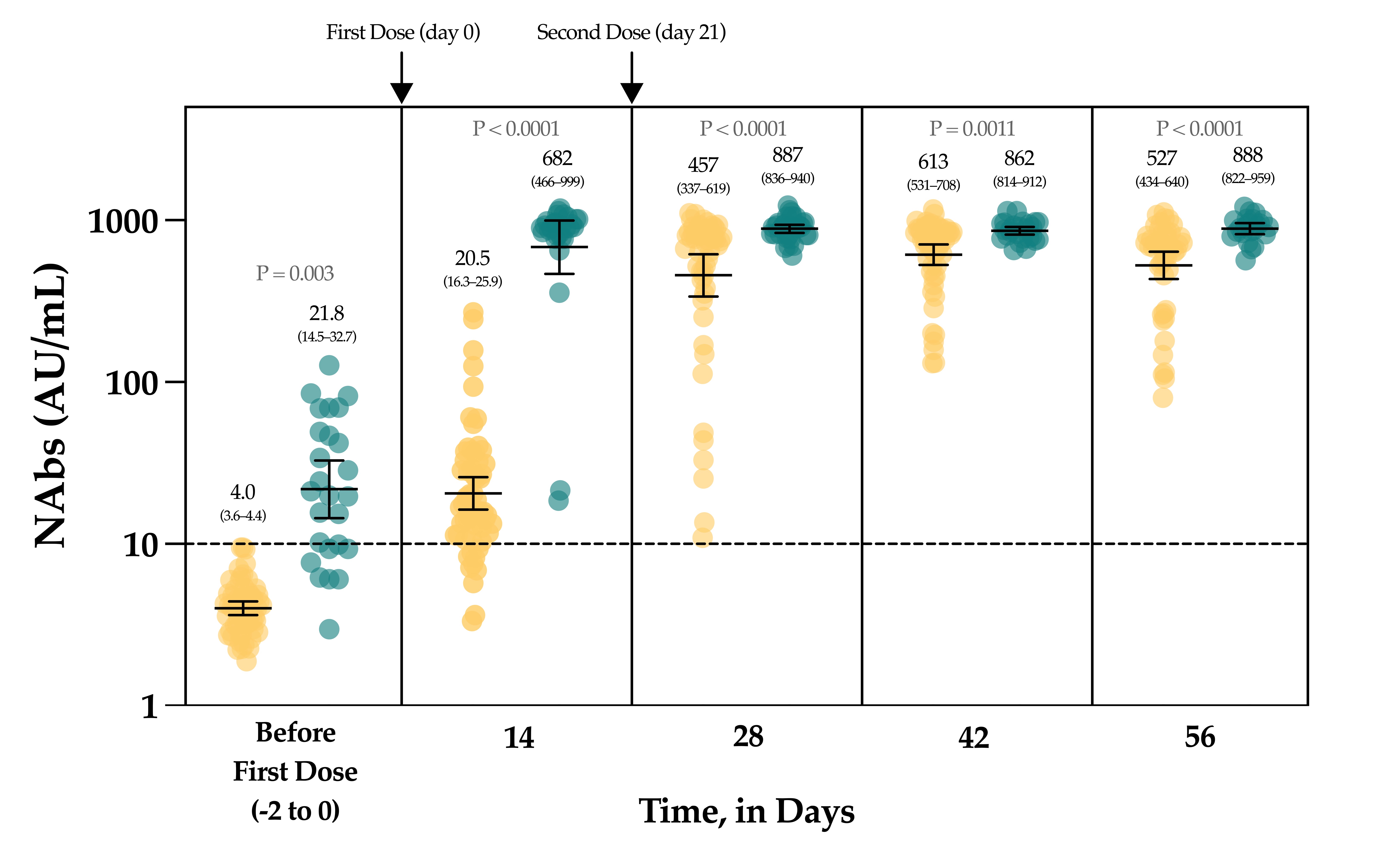
In the group of vaccinated individuals from the CRO-VAX HCP study [
38,
39,
40], we evaluated the neutralizing response in a cohort of 90 volunteers, of which 60 were uninfected and 30 were previously infected by SARS-CoV-2, having received the complete dose regimen of the BNT162b2 vaccine. NAbs were measured at baseline, i.e., just before the administration of the first dose, and at 14, 28, 42 and 56 days. So far, few reports have investigated the neutralizing response in vaccinated subjects [
41,
42,
43,
44,
45,
46] and they mainly included few participants, only investigating the effect of the first dose [
42,
43,
45], or did not include previously infected individuals [
46]. In our study, a significant increase in NAb titers was seen after the first dose (i.e., a 5.1- and a 31.1-fold increase in uninfected and previously infected individuals, respectively) in all participants (
Figure 4). Interestingly, the neutralizing capacity was similar when comparing previously infected individuals at baseline and naive individuals after the first dose, an observation that is similar to that of Manisty et al. using the Roche RBD total assay [
47]. After the second dose, a significant increase in NAb titers was only observed in uninfected individuals (i.e., a 22.3-fold increase between day 14 and 28). Afterwards, the peak of the neutralizing capacity seems to have been reached at day 42 (i.e., 613 AU/mL) and a slight but non-significant decrease was observed at day 56 (527 AU/mL), which could be explained by the natural clearance of antibodies via excretion or mostly via catabolism [
48]. Terpos et al. obtained similar findings using the cPass™ sVNT from GenScript [
46]. All participants were considered positive 7 days after the second dose. In previously infected individuals, NAb titers at days 28 to 56, i.e., 7 and 35 days after the second dose, were not significantly different from those at day 14 after the first dose (
Figure 4). The non-significant differences between the neutralizing capacity after the first dose and after the second dose support the concept only one dose might be sufficient to generate a complete neutralizing antibody response in individuals with a previous SARS-CoV-2 infection (
Figure 4). Using an sVNT, Ebiger et al. also noticed a similar response after the second dose in previously infected individuals, but the number of participants who had received the second dose was low (
n = 11) and they were followed up for a maximum of 28–42 days [
41]. Evaluation of the pre-vaccinal serological status could therefore be proposed as a strategy to identify patients who will only require the booster dose [
47]. In this context, pan-immunoglobulin assays should be preferred due to their higher sensitivity observed in long-term studies (up to 1 year post-infection) [
13,
49] compared to Nabs, which were negative in eight out of 30 (73.3%) previously infected individuals in our cohort (median days since RT-PCR = 158) (
Figure 4). The NAb titer after the first dose in previously infected individuals was not significantly different from the NAb titers of uninfected individuals after the two-dose regimen (
p-value > 0.05), even if lower mean titers were reported (
Figure 4). This finding is inconsistent with the recent data of Anchini et al., who reported significantly higher NAb titers in previously infected individuals after the first dose compared to the uninfected individuals who had received two doses [
44].
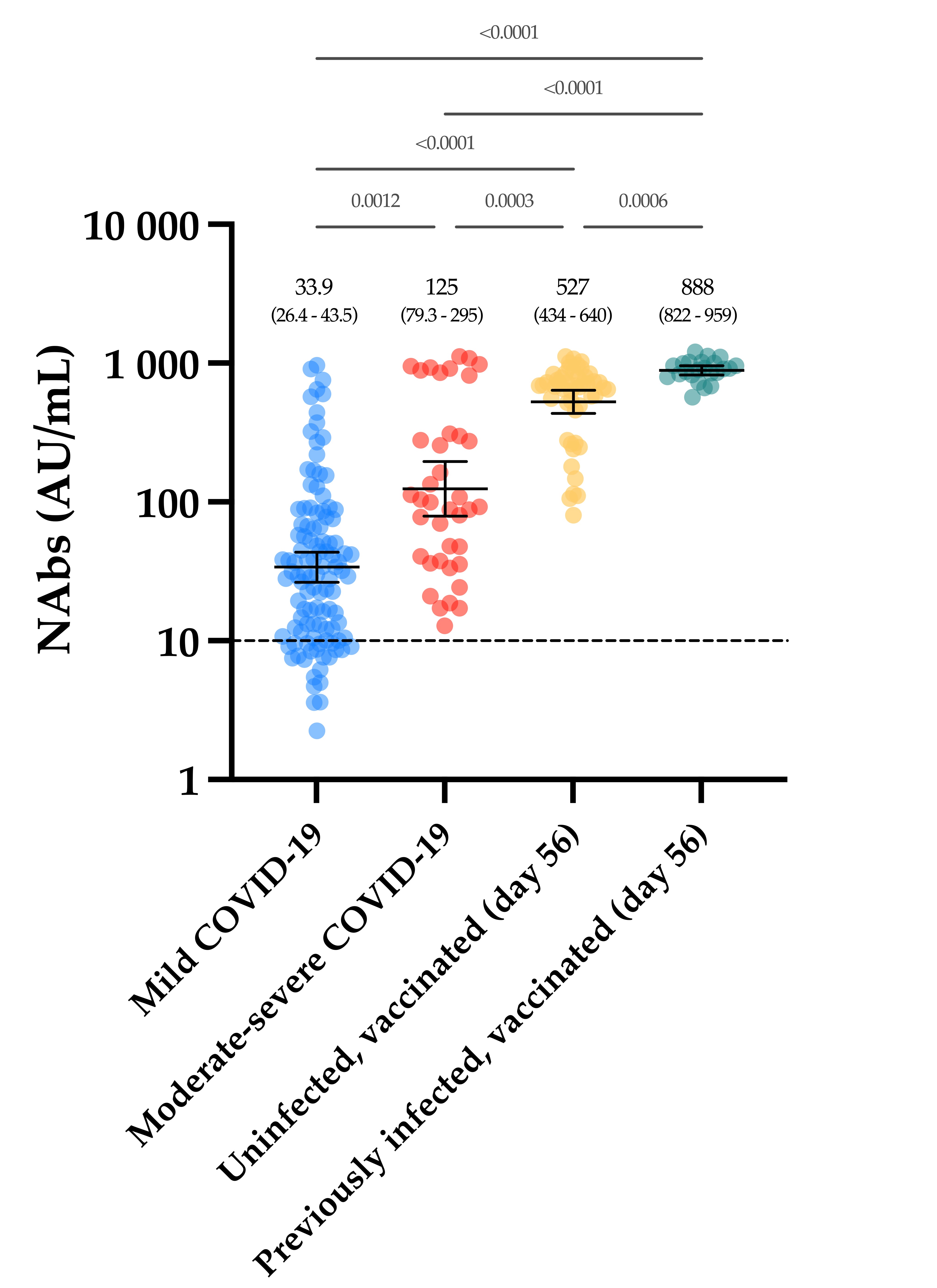
In conclusion, we found a stronger neutralizing capacity in moderate–severe versus mild COVID-19 patients, in which a slow decay with time was observed. Vaccinated participants had significantly higher NAb titers after the complete dose regimen of the BNT162b2 vaccine compared to our cohort of COVID-19 patients. In light of these data, we can hypothesize that only one dose of the BNT162b2 vaccine might be sufficient in previously infected individuals to generate sufficient NAb titers to confer a sufficient serological immunity.