Cooled preservation of semen is usually associated with artificial insemination and genetic improvement programs in livestock species. Several studies have reported an increase in reactive oxidative species and a decrease in antioxidant substances and sperm quality parameters during long-term semen storage at refrigerated temperatures. The supplementation of antioxidants in extenders before refrigeration could reduce this detrimental effect. Various antioxidants have been tested, both enzymatic, such as superoxide dismutase and catalase, and non-enzymatic, such as reduced glutathione, vitamins E and C and melatonin. However, the problem of oxidative stress in semen storage has not been fully resolved. The effects of antioxidants for semen-cooled storage have not been reviewed in depth. Therefore, the objective of the present study was to review the efficiency of the supplementation of antioxidants in the extender during cooled storage of semen in livestock species.
1. Introduction
Semen preservation, either by freezing or refrigeration allows the separation of the moment of extraction from that of use in artificial insemination (AI) or in vitro fertilization, providing multiple applications in livestock and human species [
1,
2]. In the case of livestock, semen preservation is usually associated with the AI technique and genetic improvement programs, allowing its use in places far from AI centers. AI is used for progeny testing of young males and for disseminating genetic improvement [
3]. Cryopreservation or cooled liquid storage have different pros and cons [
3], and the choice of the preservation method will depend on the AI efficiency in the specific species and the objective of the AI program. For example, frozen semen is usually used in bovine, while in porcine, AI is mainly performed with semen doses refrigerated at 15–18 °C and stored for several days. In general, fertility after AI is higher when using cooled rather than frozen/thawed semen.
In humans, spermatozoa are frozen to preserve fertility for the future (for example, prior to chemotherapy treatment [
2]) or for depositing in donor banks. Sometimes cooled preservation can be useful for transporting raw semen samples from one laboratory or collection place to another for additional tests or uses [
4]. Cooled semen is commonly used in domestic animals; therefore, the majority of the research studies concerning liquid cooled storage of semen referred to in this review were carried out with livestock species. Although freezing/thawing and refrigeration of semen are routine procedures in laboratories of livestock AI centers or human assisted reproduction clinics [
1], these procedures are not always optimized, and a worsening of several important sperm quality parameters has frequently been observed [
5,
6].
Oxidative and nitrosative stress occurs when there is an excess of oxidants (reactive oxygen species (ROS) and reactive nitrogen species (RNS)), a deficiency of antioxidants, or both [
7,
8]. When sperm samples were stored cooled for a certain time, an increase in ROS [
9,
10,
11] and a decrease in antioxidants [
11] were observed. Treatments with antioxidants to avoid damage due to oxidative stress (OS) in gametes can be approached from different perspectives [
12]. The first would be oral antioxidant supplementation, an approach widely discussed in different reviews on both male and female gametes in humans [
6,
13,
14]. The second would be the supplementation of antioxidants in media used during assisted reproductive technologies, mainly in semen extenders used to preserve samples. In this context, the use of antioxidants in the frozen/thawing process has been extensively discussed in several works [
15,
16,
17]. However, the effect of the inclusion of antioxidants in extenders used for semen cooled storage has not been reviewed in depth. Conclusions obtained in cryopreservation studies may not be applicable to refrigeration. There are substantial differences between the freezing and the refrigeration process, such as osmotic shock, cryoprotectant toxicity and/or the presence of ice crystals. In addition, cellular metabolism is practically stopped in frozen samples while in cooled storage sperm metabolism does not stop completely and the number of dead sperm progressively increases over time.
2. Treatments with Antioxidants in the Preservation Process
2.1. Enzymatic Antioxidants
Antioxidant enzymes present in spermatozoa and/or seminal plasma include SOD, CAT and GPx. As
Table 1 shows, SOD and CAT are the most extensively studied enzymatic antioxidants. SOD is the main antioxidant enzyme in seminal plasma [
15,
99] and protects the cell against O
2•—, as it catalyzes the dismutation of this anion to H
2O
2. Additionally, this reaction prevents the formation of the highly reactive ·OH which happens when O
2•— and H
2O
2 react with ferric ion by the Haber-Weiss reaction [
100]. However, SOD activity promotes the formation of H
2O
2, a more stable and long-lived ROS, which can be removed by the cell using other enzymatic antioxidants such as CAT and GPx. In general, the addition of SOD to extenders, both alone or in combination with other antioxidants, has been found to increase sperm motility and viability in comparison with control groups in several species, although in canine and ovine these effects were not always evident (
Table 1; [
9,
77,
87,
101,
102,
103,
104]). In dogs, supplementation with SOD or SOD plus GPx did not improve the majority of sperm quality parameters in comparison with the control group [
101].
Table 1. Effects of enzymatic antioxidants in liquid cooled storage on sperm parameters.
| Antioxidant |
Concentration |
Opt |
A/C |
Temp |
Time |
Species |
In Vitro Effects |
Ref. |
| CAT |
50–150 U/mL |
100 |
A |
4 °C |
30 h |
bovine |
Increased sperm motility and decreased dead or abnormal spermatozoa, and acrosomal abnormalities compared with control group. |
[79] |
| CAT |
100 U/mL |
|
A |
4 °C |
72 h |
canine |
Reduced total ROS, increased sperm motility. |
[105] |
| CAT |
90–3600 U/mL |
|
A |
5 °C |
72 h |
equine |
No effect or detrimental effect at high concentrations. |
[95] |
| CAT |
100–200 U/mL |
|
A |
5 °C |
72 h |
equine |
No effect on sperm motility. Increased viability in certain cases. |
[106] |
| CAT |
100–800 U/mL |
100/200 |
A |
5 °C |
4 d |
ovine |
Increased sperm motility only on 4th day. |
[77] |
| CAT |
100–400 mM |
200–400 mM |
A |
5 °C |
24 h |
ovine |
Slight effects on sperm motility. |
[111] |
| GPx |
1–10 U/mL |
10 U/mL |
A |
5 °C |
6 d |
ovine |
Improved sperm motility on 6th day. |
[77] |
| GPx |
1–10 U/mL |
10 U/mL |
A |
25 °C |
6 d |
ovine |
Improved sperm motility on 6th day. |
[77] |
| SOD |
50–150 U/mL |
100 |
A |
4 °C |
30 h |
bovine |
Increased motility and decreased dead or abnormal spermatozoa, and sperm acrosomal abnormalities compared with control group. |
[87] |
| SOD |
100 U/mL |
|
A |
4 °C |
96 h |
canine |
No effect with respect to control group. |
[101] |
| SOD |
25–50 U/mL |
|
A |
5 °C |
72 h |
equine |
Increased sperm motility and viability compared with control group. |
[102] |
| SOD |
100–800 U/mL |
800 U/mL |
A |
5 °C |
6 d |
ovine |
Improved sperm motility. |
[77] |
| Mix: GSH + CAT |
10 mM GSH + 100 IU/mL CAT |
|
C |
4 °C |
72 h |
ovine |
No effect on viability or total motility. Reduced MDA. |
[80] |
| Mix: Vit EP, SOD + Cat and GPx |
Vit EP 12.5 μmol/L, SOD 37 μmol/L+ CAT 500 IU/mL, and GPx 20 IU/ml |
|
C |
5 °C |
72 h |
ovine |
No effect on the studied sperm parameters (viability, acrosome). |
[9] |
| SOD, CAT and GPx |
15 IU/mL each of one |
|
C |
4 °C |
10 d |
canine |
Increased total and progressive sperm motility, reduced DNA fragmentation mainly in hypofertile males. |
[103] |
| SOD, CAT and GPx |
15 IU/mL each of one |
|
C |
5 °C |
72 h |
equine |
Increased motility and viability, and reduced DNA damage of spermatozoa compared with control group after 72 h storage. |
[104] |
| SOD + GPx |
100 and 5 U/mL respectively |
|
C |
4 °C |
96 h |
canine |
No effect in sperm motility, DNA or acrosome status with compared with control group. Increased viability. |
[101] |
The CAT enzyme catalyzes the reaction to convert H
2O
2 into water [
61]. CAT was one of the first enzymatic antioxidants to be related to OS and sperm motility in McLeod’s work in 1943 [
16], and it is the most extensively studied enzymatic antioxidant, alone or in combination, in sperm cooled storage in livestock species. As with SOD, a positive effect of CAT has also been observed in several species, mainly increasing sperm motility [
77,
79,
105]. However, no effects, or even detrimental effects, were observed in equine [
95,
106].
The GPx enzyme exerts its antioxidant actions by using the reduced form of glutathione (GSH) as an electron donor to reduce H
2O
2 to water. Because of this reaction, GSH is oxidized to glutathione disulfide (GSSG), so finally another member of the GSH family of enzymes, glutathione reductase (GR), is responsible for regenerating GSH by transferring a proton from NADPH to GSSG [
107]. Despite its importance in reducing H
2O
2, we found only one research study in the last 25 years using GPx alone in liquid cooled storage of spermatozoa [
77], and in only a few studies has it been used in combination with other antioxidants in canine, equine and ovine [
9,
101,
103,
104]. Other studies on the effect of GPx on sperm quality parameters after freezing/thawing have been reported, but without conclusive results [
108,
109,
110]. Antioxidant action of GPx is conditioned by the presence of GSH and H
2O
2 and this latter, in turn, is influenced by the presence of SOD. This is may be the reason that GPx has been studied more in combination with other antioxidant substances than alone.
2.2. Non-Enzymatic Antioxidants
2.2.1. Amino Acids and Small Peptides
The antioxidant effect of many amino acids and small peptides, such as GSH, cysteine, hypotaurine, taurine, carnitine, glutamine, proline or methionine, has been studied in refrigerated semen samples (see
Table 2). Thiols (−SH) such as cysteine, taurine, hypotaurine and GSH are a large class of antioxidants. Cysteine is, together with glutamate and glycine, one of the GSH components which supplies to this small peptide the –SH group, an essential chemical functional group for its scavenging actions. Among all of these, GSH is the most widely studied antioxidant in cooled semen, mainly in porcine, ovine and bovine. GSH is a low-molecular-weight compound made from the amino acids cysteine, glutamate and glycine. This small peptide exerts its antioxidant action in two ways: (1) directly neutralizing ROS, mainly due to the presence of the -SH deriving from the cysteine residue, and (2) maintaining other antioxidants such as vitamin C or E in their oxidized active forms. In addition, GSH protects cells by repairing damaged proteins, nucleic acids and peroxidated lipids, and maintaining a reducing state of the proteins’ sulphydryl groups [
64]. Although numerous studies have been conducted using GSH as an antioxidant in semen extenders, the results still remain controversial. On the one hand, several studies found that supplementation of the medium with GSH increased motility, kinetics, viability and T-AOC [
94,
112,
113,
114,
115,
116,
117]. However, many other studies found no effects or detrimental effects at high concentrations [
94,
111,
112,
118,
119,
120]. In rams in particular, the majority of studies found no effects of extender supplementation with GSH. It is possible that, in the case of GSH, the concentration may be determinant, and this may be variable depending on the species. In bovine and porcine, the optimal concentration seems to vary between 0.5 and 1.5 mM [
94,
112,
114], but in ovine, the studied concentrations were much higher than in other species [
115].
Table 2. Effects of non-enzymatic antioxidants (amino acids and small peptides) supplementation in liquid cooled storage on sperm quality parameters.
Another amino acid related to GSH used as an antioxidant in sperm cooled storage is cysteine. High levels of this amino acid are necessary to ensure adequate GSH levels, so under conditions of oxidative/nitrosative stress increased cysteine availability may be needed. When cysteine is oxidized, it is transformed to cystine in a reversible manner. Supplementation of cystine increased GSH and antioxidant capacity both in fresh and frozen/thawed spermatozoa [
121]. Few studies have been conducted with different species using cysteine supplementation in extenders for cooled semen storage ([
116,
122,
123]). Although in two of them, sperm motility and viability increased [
116,
123], these results are not conclusive.
Taurine, a sulphonyl amino acid derived from cysteine, and its intermediate hypotaurine have also been used as antioxidants in sperm extenders, mainly the former. In general, taurine reduced the drop of sperm motility, viability and acrosome integrity during cooled storage in several species. However, no beneficial effect was observed in ovine (
Table 2; [
91,
105,
119,
124,
125,
126]).
Carnitine is a polar compound, highly distributed along the body and particularly concentrated in high energy demanding tissues such as the epididymis [
127]. Since this compound has an important function transporting fatty acids into the sperm mitochondria, it plays a key role in sperm motility providing large amounts of energy through β-oxidation. In fact, increased motility of sperm in epididymal fluid has been related with the carnitine concentration [
128]. However, carnitine is also an effective antioxidant, which (1) reduces lipid availability for peroxidation by allowing fatty acids to cross the mitochondrial membranes, (2) prevents OS protecting the antioxidant enzymes CAT, SOD and GPx from further peroxidative damage, and (3) has a direct scavenging action of FRs like O
2•— or H
2O
2 [
129]. Carnitine has been used as an antioxidant in some studies in equine, porcine and rabbit, maintaining sperm quality parameters such as motility, viability and acrosome integrity during cooled storage (
Table 2; [
128,
130,
131,
132]). The carnitine concentration used in these works was variable, even in the same species.
Finally, other amino acids such as glutamine and proline generally showed beneficial effects, increasing sperm motility, viability and reducing ROS during cooled storage compared to control groups (
Table 2). In this regard, glutamine is an amino acid precursor of GSH, and proline has antioxidant properties based on its secondary amine structure [
133]. Methionine is an amino acid capable of protecting cells from oxidative damage by acting as a precursor amino acid for cysteine, and also due to its capacity to react with oxidants to form methionine sulfoxide [
134]. Several studies have been developed to evaluate the effect of glutamine, with these showing beneficial effects (
Table 2; [
135,
136,
137]).
2.2.2. Vitamins, Carotenoids and Polyphenols
Vitamin E, vitamin C, polyphenols and carotenoids are all well-known natural antioxidants [
139] which have been used to supplement semen extenders to palliate the detrimental effect of cooled storage (
Table 3). The term vitamin E refers to a set of tocopherols (α, β, γ, δ) and tocotrienols (α, β, γ, δ). Among them, α-tocopherol is the most potent lipid-soluble antioxidant, and can block the LPO reaction chain by donating an electron to a lipid- or a lipid hydroperoxide radical, transforming itself into the relatively stable tocopheroxyl radical. The latter can be transformed back to the active tocopherol form by reacting with other antioxidants such as vitamin C or GSH [
6,
13]. Trolox is a synthetic water-soluble vitamin E analogue. The effect of vitamin E, both as α-tocopherol or Trolox, has been extensively studied in cooled semen in several livestock species (
Table 3; [
105,
106,
113,
118,
140,
141,
142]). However, most studies did not find a beneficial effect on sperm quality parameters.
Table 3. Effects of non-enzymatic antioxidants (vitamins, phenols, indoles and other types) supplementation in liquid cooled storage on sperm quality parameters.
Vitamin C is a hydrosoluble antioxidant due to its ability to function as a reducing agent, which can donate one or two electrons and oxidize itself to the ascorbyl radical or dehydroascorbic acid (DHA), respectively. Later, DHA can be reconverted back to the reduced form at the expense of GSH oxidation to GSSG. Antioxidant actions of vitamin C include both a direct scavenging action of a wide range of RONS including hydroxyl, superoxide and peroxynitrite radicals, and an indirect scavenging action of lipophilic radicals by reducing the tocopheroxyl radical to its active form tocopherol [
155]. As with vitamin E, the supplementation of vitamin C to the semen extender did not exert a significant beneficial effect on sperm quality parameters during liquid cooled storage (
Table 3; [
94,
105,
106]). Only Aurich et al. [
95] found vitamin C supplementation increased sperm viability, although it was toxic at high concentrations.
Lastly, polyphenols such as resveratrol, quercetin, procyanidin, hydroxytyrosol and 3,4-dihydroxyphenylglycol have been used as antioxidants in semen cooled storage (
Table 3; [
88,
136,
149,
150,
151,
152,
153,
154]. Polyphenols are secondary plant-derived metabolites characterized by multiple phenol units. These compounds are structurally very diverse and include four principal classes: phenolic acids, flavonoids (such as quercetin), stilbenes (such as resveratrol) and lignans. Because of their chemical structure, these compounds are natural antioxidants that tend to oxidation, which allows them to intercept FRs and protect cells from oxidative damage. Moreover, some polyphenols have an enzymatic antioxidative action since they can upregulate antioxidant enzymes [
6,
156]. Among the numerous polyphenols studied, resveratrol has been the most frequently used, mainly in porcine semen [
150,
151,
152,
153,
154]. However, the results are not conclusive, with more studies showing no effects than beneficial effects. It seems that the resveratrol concentration may be important (optimal concentration around 50 µM), since several studies have observed detrimental effects at high concentrations [
150,
152,
153].
2.2.3. Other Antioxidant Substances
Some hormones, including melatonin, show antioxidant properties [
157]. Melatonin is a tryptophan-derived indole synthesized and secreted by the pineal gland during the night. In addition to its role in the regulation of the circadian cycle or seasonal reproduction in mammals, this hormone has significant antioxidant functions. Receptors of melatonin have been found in human, hamster and ram spermatozoa, in addition to their presence in the seminal fluid [
158,
159,
160], but not in stallion sperm [
161]. Melatonin has demonstrated both a direct antioxidant action scavenging some RONS, such as ·OH, O
2•−, ONOO
− and ·NO [
162], and an indirect antioxidant action by stimulating the activity of endogenous antioxidants such as CAT, SOD or GPx [
157,
163]. Melatonin has been used as an antioxidant in extenders in several studies in bovine, ovine, porcine, rabbit and equine, with a predominant increase in sperm motility, viability and reduced LPO with respect to control groups (
Table 3 [
83,
93,
143,
144,
145,
146,
147,
148]). Semen incubated with melatonin at 37 °C maintained or improved quality sperm parameters and increased blastocyst rate after in vitro fertilization respect to control group [
163]. Moreover, melatonin reduced detrimental effects of H
2O
2 on sperm parameters and in vitro embryo production [
163].
Finally, Lycopene, a red carotenoid found in fruits and vegetables such as tomatoes, carrots or grapefruits, has been described as a potent antioxidant with an efficacy two times superior to that of β-carotene and 10 times that of α-tocopherol [
164]. Its antioxidant actions are attributed mainly to its chemical structure. Lycopene has been confirmed as able to scavenge ONOO
−, nitrogen dioxide as well as thiol and sulphonyl radicals [
61,
165]. Recently, Sheikholeslami et al. [
90] observed that the addition of lycopene to an extender increased motility and viability and reduced the LPO of dog sperm compared to the control group (
Table 3).
3. Treatments with Antioxidants in Livestock Species
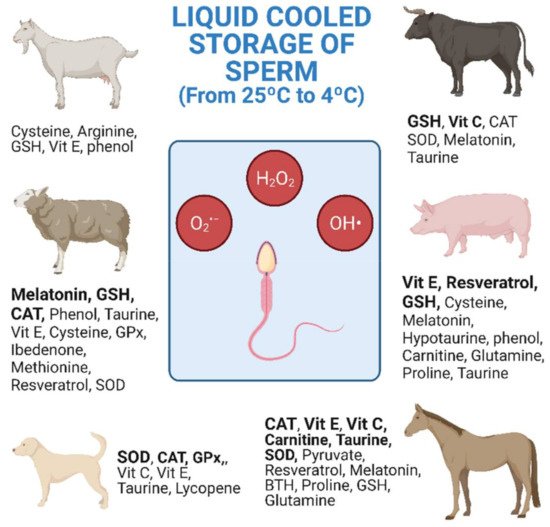
The research studies shown in Table 1, Table 2 and Table 3 are collected and distributed by animal species in Figure 1. The figure shows the antioxidants used, as well as the number of investigations carried out according to the animal species. Equine and ovine, closely followed by porcine, are the animal species for which the greatest number of studies of antioxidants in cooled semen have been carried out (Figure 1).
Figure 1. Antioxidants used in cooled semen storage by species. Research studies listed in Table 1, Table 2 and Table 3 collected together and distributed by animal species. Bold: 2 or more different research studies carried out (both alone and in combination) (Created with BioRender.com).
In general, cooled semen has a greater longevity in the female tract than frozen semen [
166]. This means that AI with frozen semen must be more precise for both the deposition place and ovulation time [
166]. In sheep, the explanation for the great interest in improving cooled storage may be due to various reasons including the difficulty of carrying out post-cervical AI and the low longevity of the refrigerated semen. The cervix of the sheep is intricate and post-cervical AI is more difficult than with other species such as cattle or goats. In ovine, the most widely investigated antioxidants are CAT, GSH and melatonin, the latter giving the best results with refrigerated semen (
Figure 1 and
Table 3 [
144,
145,
147]). In equine, AI with refrigerated semen is easier and achieves higher pregnancy rates than when using frozen–thawed semen [
167]. Moreover, semen doses from valuable stallions reach high prices in the market [
168]. In stallions, CAT, SOD, GSH, vitamin E, carnitine and taurine are the most investigated antioxidants. However, only the amino acids showed repeated beneficial results on sperm motility with refrigerated semen (
Figure 1 and
Table 3 [
125,
126,
130,
132]). In pigs, although semen freezing was developed many years ago, AI with refrigerated semen is still by far the most commonly used method [
169]. In this species, the most studied antioxidants are GSH, vitamin E and, more recently, resveratrol, but without achieving clear conclusive results for cooled semen storage.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10071096