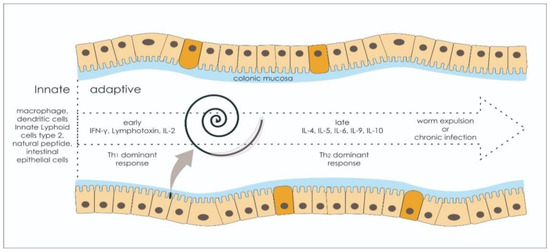
ESPs are a group of molecules released from helminths, bearing immunomodulation features during the host–parasite interaction consisting of some uncharacterized substances, proteases, glycolytic enzymes, protease inhibitors, chaperones, miRNA, and antigen homologs or metabolites [
30,
83,
84]. Whipworms organize the substances for survival and modulate the immune response through two primary mechanisms. First, ESPs can manipulate the expression of PRRs or act as cytokine homologs, affecting downstream signaling pathways [
85]. Second, immunomodulation features can also trigger several changes in the response to the regulation and polarization of immune cells that could blunt inflammatory responses via anti-inflammatory cytokine secretion, such as IL10 and transforming growth factor β (TGFβ). In a study, the direct effect of the presence of ESPs reduced IL-1β, TNF-α, and NO-2 as secreted products of macrophages in the large intestine [
31]. However, whey acidic protein (WAP), as an abundant type of EPs found in
T. muris, induces induce type-2 immunity that eventually promotes worm expulsion [
86].
2.1. Secreted Products Modulate Pattern Recognition Receptor (PRR)
ESPs impair the function of PRR, which is an essential part of the innate immune response for antigen recognition. There are several types of PRRs, such as retinoic-acid inducible gene (RIG)-like receptors (RLRs), NLRs, CLRs, and toll-like receptors (TLRs), contained by future antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs). By modulating the activation of PRRs, the ESPs of
T. suis were shown to prevent lipopolysaccharide-induced TLR4 sensing in human dendritic cells (DCs) in a study, suppressing downstream signaling pathways [
87]. It was also evident that
T. suis SPs transform macrophages into a more anti-inflammatory phenotype by inhibiting P2RX7, a receptor involved in the stimulation of immune cells such as macrophages, dendritic cells, and lymphocytes, concurrent with reduced IL12B, CCL1, and CXCL9 expression [
88]. This interaction reduced the expression of proinflammatory cytokines via Rab7b overexpression, a small GTPase-degrading TLR4 [
89,
90]. Both pathways related to TLR4 for myeloid differentiation would undoubtedly also be impaired. Reductions mainly involve MyD88-dependent mediated TLR4 responses, finally reducing the level of proinflammatory cytokine genes, reactive oxygen species (ROS), and eicosanoids [
89]. ESPs also affected the remaining TLR4 signaling pathway in a TIR-domain-containing adaptor protein-inducing, interferon-β (TRIF)-dependent manner, by reducing IFN α/β production, finally resulting in the scarcity of expression of type I IFNs [
91].
Retinoic acid-inducible gene (RIG)-I-like receptors also disintegrate following ESP administration, downregulating several essential signaling proteins such as lrf7, Ddx60, and Dhx58 [
92]. Thus, there is a decreased downstream signaling for proinflammatory cytokines, and the threats for type-I IFN production become more prominent [
93].
Impairing TLR4 activation prevents the surge of proinflammatory cytokine secretion, which eventually results in several clinical implications for other conditions. In sepsis, the hyperactivation of TLR4 and TLR2 is concurrent with the overload of systemic inflammation and organ dysfunction and could produce poor outcomes in animal models [
94].
Trichuris infection prevents TLR4 activation by downgrading its receptor and signaling pathway, thus reducing the repercussions of proinflammatory cytokine upregulation [
88]. Additionally, the immunomodulation properties of
Trichuris could also increase insulin sensitivity [
95]. Dietary fatty acids and enteric lipopolysaccharides (LPS) can activate TLR4 and provoke proinflammatory responses to behave as insulin resistance inducers [
96,
97]. Therefore, preventing TLR4 signal activation could be the novel target to increase insulin sensitivity.
Glycan-based components in
Trichuris sp. SPs bind the mannose receptor, a CLR, and increase its expression in monocytes and dendritic cells, inducing protein kinase C (PKC) phosphorylation, specifically PKC δ, and shift the monocyte behavior to an anti-inflammatory phenotype [
98,
99]. Most novel PKC activation implications remain unknown, but CC chemokine receptor (CCR) 2 and lymphocyte function-associated antigen (LFA) 1 expression are upregulated following PKC activation [
100,
101]. In IBD, a disease characterized by gut physiology resembling
T. muris infection, breaking mucosal integrity becomes the basis of pathogenesis, which is perpetually insulted by the expression of proinflammatory cytokine and oxidants caused by the PKC downstream signaling pathway [
102]. Moreover, PKC inhibitors were found to attenuate tissue injury in a mice model for colitis [
103]. The same study also suggested a breakthrough in advancing qualities for managing several autoimmune diseases with T cells and the autoreactivity of monocyte-derived macrophages using
Trichuris sp.-secreted products, such as multiple sclerosis and IBD [
98].
Cytosolic PRR or the inflammasome have demonstrated several pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [
85]. The presence of ESPs and other extracellular vesicles released from
Trichuris also affects the function of NOD-like receptor protein 3 (NLRP3), a well-known inflammasome that has pivotal roles during the initiation and amplification phases of both the innate and adaptive immune response [
104].
Trichuris exosomes encourage pro-helminthic immunity by upregulating IL1β and IL18 via the NLRP3-dependent pathway [
104,
105]. IL18 appears to be a driving force for the different outcomes since it has a diverse function that could initiate a resistant or susceptible type of immune response. IL18 used to be known as IFNϒ-inducing factor (IGIF) and is involved in the vast signaling pathway for Th1 and NK cell activation, but in vivo studies have suggested that IL18 undermines anti-helminth immunity through an IFNϒ-independent pathway. In other studies, NLRP3 activation triggered downstream signaling pathways of the Th1-type response, making the host susceptible to chronic infection [
104]. In contrast, infecting NLRP3-deficient mice with helminths augmented early innate immune cell recruitment, eosinophilia, and neutrophilia, as well as type-2 cytokine responses, while the presence of NLRP3 attenuated immunopathological changes in the tissue environment [
106].
2.2. Secreted Product Skewed Innate Immune System
Trichuris ESPs and SPs have also become key to developing the innate immune system by shifting the response of classical (inflammatory type) into nonclassical monocytes after
Trichuris SPs administration. This type of monocyte has no expression of CCR2 and CD14 but has a higher expression of CX3CR1. A group of proinflammatory cytokines (a marker of classical monocytes), including IL-10, TGFβ, TNFα, IL-6, and ROS, was first secreted during early observation. However, transition occurred following 16 h of SPs treatment with prominent anti-inflammatory cytokine expression, showing that classical monocytes were largely impacted by the presence of SPs [
98].
Monocyte hypermotility, mediated by the activity of small Rho GTPases such as Rho, Rac, and Cdc42 on the actin cytoskeleton, was also notable and reduced adhesion to endothelial cells following
Trichuris ESPs treatment [
98]. A high-saturated prostaglandin E2 content of EPs and SPs also manipulated dendritic cells by skewing proinflammatory features by upregulating RAB7B [
90]. PGE2 synthesis by
T. suis was independent of cyclooxygenase activity in the study. SPs modulate DCs through a mechanism that predominantly involves the overexpression of PGE2, although its effect differs based on its concentration and bound receptors [
84]. This could resolve inflammation to accommodate immunopathological repair. However, the glycan component of SPs was also found to interact with CLRs in human DC, with the final result of modulating DCs to suppress proinflammatory responses, but this is a concentration-related effect.
2.3. Secreted Product Produce Deviant Cytokine Response
ESPs influence proinflammatory cytokine expression. However, higher levels of IL10 and other regulatory cytokines, TGFβ and IL-35, are associated with the administration of
Trichuris ESPs. This also suggests that this cytokine concoction yields the main immunomodulation properties of ESPs. The regulatory function of IL10 cannot be described solely as pro- or anti-inflammatory cytokines because of the pleiotropic features caused by heterogeneous receptors IL10Rα, IL10Rβ, IL22Rα, and IL28Rα with diverse implications. Nevertheless, IL10 still augments the Th2-type immune response against acute
Trichuris infection via IL10Rα activation [
107]. It also plays a vital role in protecting the intestinal barriers, preventing other bacterial invasions, and constraining the systemic inflammatory response against
Trichuris infection. Conversely, several significant findings regarding tissue rupture are caused by
Trichuris invasion promoting bacterial translocation through the lesion. In two separate studies, the response of
T.suis inoculation was clearly found to lead to macro-pathological changes associated with bacterial infiltration and the suppression of local immunity to the
Trichuris sp. site of infection [
31,
108].
In contrast, it was observed that Alzheimer’s transgenic mice infected with
Trichuris were more vulnerable to suffer from exacerbations caused by neuroinflammation and larger microglia size, suggesting that a systemic response also developed during overwhelming IL10 secretion [
109]. Nevertheless, IL10 upregulation following ESP treatment showed positive implications in other pathological conditions, including IBD. IL10-deficient mice developed chronic inflammation, resulting in the significant immunopathology caused by an incessant immune response against normal intestinal flora [
110]. Meanwhile, the reduction of airway responsiveness and IgE production dependent on IL10 was also evident in the mice model for allergic disease, showing the immunoregulatory function of IL10 [
111]. Concerning this evidence, ESPs ultimately reduces the inflammatory response and its immunopathology by promoting IL10 secretion.
The administration of ESPs also thwarts Th2 immune cell polarization via the direct activity of ESPs modulating IL4 and IL13 expression, which are useful in different pathways for Th2 immune maintenance [
112,
113]. This recent finding shows that the protein component of ESPs secreted during chronic
T. muris infection, called p43, acts as a homolog for IL13 receptor α2 (IL13Rα2) and thrombospondin type 1 [
113]. This protein binds into the IL13 active site, inhibiting downstream activation, thus resulting in susceptibility and failed worm expulsion. In predominant eosinophilic diseases, IL13 plays a crucial role in eosinophil priming and proliferation, causing more damage to the tissue environment. Since ESP mimics IL13Rα2, it could halt the vicious pathological changes mediated by IL13 activity, such as airway hyperresponsiveness and goblet cell proliferation, as well as mucus secretion. Therefore, it might be beneficial for asthma, atopic dermatitis, and chronic rhinosinusitis with nasal polyps.