Seaweeds are heavily loaded with potential reservoirs of bioactive compounds such as polysaccharides, MAAs, natural pigments, phenolic compounds, proteins, peptides, and others. Previous studies have also investigated the anti-photoaging properties of bioactive compounds from seaweeds. In addition, further research has been carried out on the most studied seaweed-derived bioactive compounds and extracts as potential anti-photoaging agents.
3.1. Polysaccharides Rich Extract
The photoprotective activity of polysaccharides rich extract from brown seaweeds (
Hizikia fusiforme, Sargassum fusiforme, Sargassum vachellianum, and
Ecklonia maxima) was investigated and monosaccharide analysis showed that most of its rich extract contains sulfate group and a high amount of fucose (43.20 to 53.53 %) (). In addition, it was found that fucose-containing sulfated polysaccharides possessed various bioactivities and most of the polysaccharides rich extract were able to inhibit ROS production and down regulated MMP expression (especially MMP-1) [
21,
45,
46,
47,
48,
49]. This suggests that the anti-photoaging activity of polysaccharides rich extract from brown seaweeds was mainly mediated through antioxidant and MMP inhibitory activity.
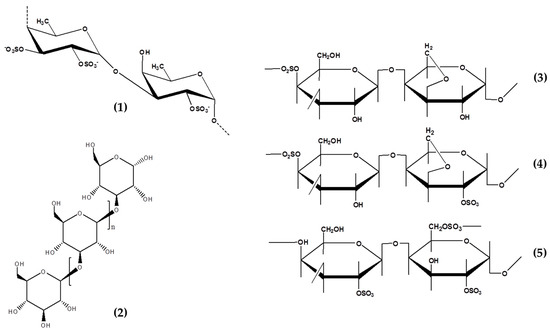
Table 2. Composition of seaweed rich polysaccharides extract showing anti-photoaging activity.
The anti-photoaging properties of two fucoidan-rich seaweed extracts from
Undaria pinnatifida and
Fucus vesiculosus have been demonstrated. Both brown seaweeds extracts showed inhibitory activity against enzymes related to skin aging process. Clinical testing showed that both extracts were able to protect skin from UVR and wrinkle depth reduction. In addition,
F. vesiculosus extract which contain polysaccharides and high polyphenol demonstrated additional efficacy in antioxidant and skin brightening benefits [
50]. In addition, the mixture of fucose and rhamnose in skincare formulation has been claimed to inhibit skin ageing process [
51].
Polysaccharides rich extracts from brown seaweeds are potentially developed as anti-photoaging agents in skincare or cosmetic products. Furthermore, when added in skincare or cosmetic products formulations, they improved the efficacy and maintained the skin in good condition especially due to their moisturizing properties. It is believed that some polysaccharides might also improve the stability and sensorial properties of cosmetic and skincare products.
3.2. Fucoidans
Fucoidans, sulfated polysaccharides, have been isolated from different brown seaweeds species. These compounds have attracted great interest in the food and cosmetic industries [
16]. Furthermore, there are many studies that focused on the isolation, characterization, and medicinal values of fucoidans and the anti-photoaging properties of fucoidan.
The antioxidant activity of fucoidan has been determined by several radical scavenging methods and the most common are 1,1-diphenyl-2-picryl hydrazil (DPPH), superoxide anion, and hydroxyl radical scavenging assays. Fucoidan have exhibited both primary (chain-breaking antioxidants) and secondary (radical scavengers) antioxidants. The primary antioxidant potential of fucoidan is characterized by its ability to react directly with free radicals and convert them to more stable non-radical products [
52,
53,
54]. Furthermore, the strong secondary antioxidant potential of fucoidan extracted from
Sargassum binderi,
Sargassum spp, and
Undaria pinnatifida has been reported [
55,
56,
57]. Its antioxidant activities are strongly related with sulfate content and molecular weight (MW). However, low molecular weight (LMW) fucoidan has shown more antioxidant potentials compared to synthetics antioxidant (Butylated hydroxyanisole; BHA) and higher MW fucoidan. Koh et al. (2019) suggested that the sulfate groups in the LMW fucoidan are more accessible compared to the ones with high molecular weight (HMW), thereby resulting in remarkably higher secondary antioxidant activity.
The photoprotective activity of fucoidan has been studied using UVB irradiated HaCaT and human foreskin fibroblast (HS 68) cells, zebrafish, and in vivo models [
19,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67]. The earliest study on the photoprotective activity of fucoidan was carried out by Kim et al. in 2008. They demonstrated its photoprotective activity in UVB-irradiated HS 68 cells via MMP-1 inhibition and ERK pathways [
58,
60]. Furthermore, the photoprotective activity of fucoidan from
Hizikia fusiforme was observed in UVB-induced photodamage in human dermal fibroblasts (HDF) cells and zebrafish models. Its treatment significantly inhibited collagenase and decreased the intracellular ROS levels. Furthermore, it significantly inhibited intracellular collagenase, reduced the expression of MMP, and improved collagen synthesis in UVB-irradiated HDF [
68]. The summary of the potential photoprotective activity of fucoidan is shown in . Fucoidan is extensively explored for its photoprotective properties and being isolated from several brown seaweed species such as
Costaria costata, Fucus evanescens, Sargassum hemiphyllum, Sargassum horneri, Sargassum siliquastrum, Ecklonia cava, Saccharina japonica, and
Hizikia fusiforme. The biological activities are affected by many factors such as seaweed species, MW, purity, sugar composition, sulfation degree, co-extracted impurities, glycosidic linkage, and branching site [
69]. In addition, it was found that the bioecology and harvesting months/seasons also influenced the composition and biological activities [
70]. Mak et al. (2013) studied the monthly variations of fucoidan content in
U. pinnatifida and it was found that the sporophyll part of
U. pinnatifida consistently contained the highest amount compared to the frond part. Furthermore, it was found that the sporophyll maturation of
U. pinnatifida strongly affected the fucoidan content and composition [
70].
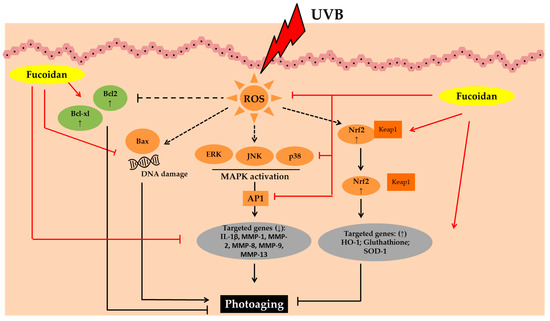
Figure 3. Photoprotective activity of fucoidan. Abbreviations: B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-extra-large (Bcl-xL), Bcl-2-associated X protein (Bax), Reactive oxygen species (ROS), Mitogen-activated protein kinases (MAPK), c-JUN N-terminal kinase (JNK), Extracellular signal-regulated kinase (ERK); Activator protein 1 (AP1), interleukin-1β (IL-1β), Matrix metalloproteinase (MMP); Nuclear factor erythroid 2–related factor 2 (Nrf2), Kelch Like ECH Associated Protein 1 (Keap1), Heme oxygenase-1 (HO-1);Superoxide dismutase 1 (SOD-1); Down-regulated, decreased (↓); up-regulated, increased (↑).
Generally, this polysaccharides contains sulfate, fucose as the main sugar, uronic acids, acetyl groups, protein, and other monosaccharides (such as mannose, glucose, galactose, xylose, and rhamnose) [
71,
72]. The structures and monosaccharide compositions of fucoidans from different brown algae sources vary from different species. Recently, Ponce et al., (2020) provided a comprehensive study on the compositional data of fucoidans from different brown seaweeds species. Its monosaccharide composition is strongly related with taxonomic classification and an example includes polysaccharides extracted from the genus Fucus which are classified as being rich in fucose (>70% of monosaccharides). Meanwhile, in order Laminariales, the presence of sulfated galactofucans with high galactose content is almost equal to the fucose content [
72]. The composition and sulfation degree of fucoidan is strongly affected by extraction and purification methods. Therefore, there is a need to develop suitable extraction techniques to maintain its composition and sulfation pattern in order to obtain the desired bioactivity [
73,
74].
Lower molecular weight has been reported to enhanced the biological activity of fucoidan [
16]. Therefore, in order to obtain LMW and stronger bioactivities; chemical, radical, acidic, and enzymatic hydrolysis are generally used. Previous studies have shown that treatment with LMW fucoidan shows stronger photoprotective activity than HMW [
62,
63,
64]. Hwang et al. (2017) provided detailed a extraction process and characterization of photoprotective activity of HMW, LMW, desulfated, and acetylated fucoidan isolated from
S. hemyphyllum () [
62]. Furthermore, LMW fucoidan showed stronger protection against UVB-induced HS 68 cells. These results suggest that its fucose content, sulfation, and MW play an important role in photoprotective activity. Supporting these results, Kim et al. (2018) stated that LMW fucoidan treatment inhibits photoaging by enhance antioxidant and anti-inflammatory activities and inhibiting extracellular matrix degradation in UVB-irradiated HR-1 (hairless) mice. It is mostly absorbed prior to UVB irradiation. Therefore, it is assumed that LMW fucoidan may involve in photoprotective effects rather than UV filtering. The LMW fucoidan extracted from
S. horneri showed a stronger photoprotective activity compared to HMW in UVB-irradiated HaCaT cells [
64].
Table 3. Sulfate, fucose, and average molecular weight of fucoidan showing photoprotective activity.
Pozharitskaya et al. (2019) investigated the pharmacokinetics of fucoidan after topical application in rats. It was found that ointment contains 15% fucoidan are distributed into the skin, striated muscle, and plasma with area under concentration-time curve for topical dose (AUC)
0–48 = 0.94 µg·h/g, 2.22 µg·h/g, and 1.92 µg·h/mL, respectively [
75]. The longest half-life for fucoidan was observed in plasma, striated muscle and skin. In addition, its accumulation in plasma was not observed after repeated topical applications of 100 mg/kg for five days. Collectively, it may be assumed that topical treatment with cream containing fucoidan have efficacy and safety benefits with little concern of accumulation and toxicity. In addition, these results suggest the potential of fucoidan as an anti-photoaging agent in skincare and cosmetic industries.
3.3. Carrageenans
Carrageenans are natural polysaccharides extracted mainly from red seaweeds (i.e.,
Eucheuma spp,
Chondrus crispus (Irish moss), and
Gigartina stellate). They are joined by α-1, 3 and β-1,4 glycosidic linkage by alternate units of d-galactose and 3,6-anhydrogalactose [
76]. Twenty percent of carrageenan production are used in pharmacy, skin care, and cosmetics products, and this is due to their unique physical functional properties (i.e., thickening, gelling, emulsifying, and stabilizing properties) [
77]. The tree main types of commercially available carrageenan include kappa (κ; forms strong, rigid gels in the presence of potassium ions), iota (ι; forms soft, clear, and elastic gels in the presence of calcium ions) and lambda (λ; does not form gel and normally used to thicken dairy products) [
17].
In addition to their thickening and gelling properties, carrageenans have also shown potential antioxidant activities. De Souza et al. (2007) tested the antioxidant activity of κ, ι and λ carrageenan and based on radical scavenging assay, λ carrageenan had better results [
78,
79]. Furthermore, it was found that the degradation into carrageenan oligosaccharides enhanced its antioxidant activity [
80]. Previous studies have shown that polysaccharides with LMW had stronger antioxidant activity compared to HMW polysaccharides. These activities may be related to the ability of LMW polysaccharides to have more reductive hydroxyl group terminals which further affect the ability to accept and eliminate free radicals. In addition Sun et al. (2015) reported that the antioxidant activities of carrageenan oligosaccharides could be related to the sulfate group, the degree of polymerization, the reduction of sugar, and the structure of reducing terminus [
81].
Thevanayagam et. al. (2013) stated that the photoprotective effects of κ-, ι- and λ-carrageenan in UVB-irradiated HaCaT cells [
82]. All carrageenan types tested in their study showed significant protection against detrimental effects of UVB-induced apoptosis in HaCaT cells and scavenge free radicals. In addition, many studies have investigated the antioxidant activities of carrageenans [
80,
81,
83,
84].
In addition, the anti-photoaging activity of carrageenan also correlates with the modulations of inflammatory responses. These polysaccharides are able to induce the activation of proinflammatory mediators such as of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, inducible nitric oxyde synthase (iNOS), and cyclooxygenase-2 (COX-2) [
85]. Furthermore, Tripp et al. (2003) found that COX-2 expression is an important factor for keratinocyte survival and proliferation after acute UV irradiation. The inhibition of COX-2 expression has been demonstrated to reduce epidermal keratinocytes proliferation [
86]. Therefore, it is believed that the modulation of inflammatory responses and antioxidant activities of carrageenan may play an important role in their anti-photoaging activity. Purwaningsih et al. (2015) formulated a sunscreen cream with carrageenan and black mangrove fruit (
Rhizopora mucronata). It was found that a sunscreen formula containing 0.5% carrageenan and 1%
R. mucronata extract showed high photoprotective properties compared to other formulas tested in their study [
87].
The photoprotective activities of carrageenan reported in previous study might reflect its new potential in skin care and cosmetic industries rather than just being used as an excipient. There are numerous advantages of these polysaccharides over other bioactive substances, including relatively low production costs, safety, non-toxic properties, wide acceptability, suggesting carrageenan as a promising anti-photoaging candidate in the near future; however, further studies such as formulations in order to obtain the most optimum anti-photoaging properties are required.
3.4. Laminarins
Laminarins are storage polysaccharides extracted from brown seaweeds and composed of (1–3)-β-d-glucan with β-(1–6) branching with different reducing endings either mannitol or glucose residues. Laminarin has been extracted from several brown algae species such as
Eisenia bicyclis, Saccharina longicruris, Laminaria digitata, Laminaria hyperborean, Laminaria japonica, Sargassum mcclurei, Cystoseira barbata, and
Durvillaea potatorum [
88,
89,
90,
91].
In vivo studies have shown the anti-photoaging potential of laminarin and an example is the study conducted by Li and colleagues (2013) which was based on the effect of laminarin on the activity of MMP-1 of photoaging skin in mice models. The laminarin treatment significantly increased the thickness of dermis, tissue inhibitor MMP-1 (TIMP-1) level, and decreased the expression and release of MMP-1 [
92]. It also protected mouse dorsal skin from UVB induced photodamage [
93]. Furthermore, it significantly increased collagen fibers in the dermis of the UVB treated ICR mice. Laminarin pretreatment provided photoprotection by decreasing oxidative stress and increasing antioxidant enzymes including superoxide dismutase (SOD)-1, SOD-2, glutathione peroxidase (GPx), and catalase (CAT). In addition, it also showed photoprotective properties in UVA-irradiated HDF, HaCaT and normal human epidermal keratinocytes (NHEK) cells [
89]. Treatment with laminarin attenuates pro-inflammatory cytokines (IL-6) levels and basal ROS levels in HDF and NHEK cells at concentration of 1 and 10 µg/mL.
Many studies have reported the enhanced antioxidant activity of LMW laminarin [
18,
90,
94,
95,
96,
97,
98]. This encouraged Choi et al. (2011) to prepare LMW laminarin by gamma irradiation and the formation of carbonyl groups by gamma irradiation was observed. Carbonyl groups were mainly attributed to the enhanced antioxidative activity of laminarin [
95,
96]. However, Rajauria et al. (2021) found that the purification of laminarin which involve solvents and molecular weight cut-off (MWCO) filters reduced the antioxidant activity compared to the crude laminarin extract [
94]. In addition, chemical modifications (i.e., sulfation, carboxymethylation, acetylation, phosphorylation, and benzoylation) have affected the antioxidant activity of polysaccharides to some extent. The chemical modifications of laminarin via carboxylation using dielectric barrier discharge, conjugation with gallic acid, and sulfation have also been reported. Analyses of the chemical composition of carboxylated laminarin (LMC), gallic acid-conjugated laminarin (LMG), and sulphated laminarin (LMS) yielded 11.7% carboxyl groups, 1.5% gallic acid, and 1.4% sulfate content, respectively. This chemically modified laminarin was tested against several antioxidant assays including total antioxidant, hydroxyl radical scavenging, superoxide radical scavenging, iron chelating, reducing power and copper chelating assays. It was reported that LMG showed better antioxidant activities compared to other chemically modified laminarin [
98].
Interestingly, Sellimi et al. (2018) showed that the topical application of laminarin-based creams improved the wound healing process in rats by accelerating the collagen deposition and re-epithelization and protected the skin cells from oxidative stress [
91]. It appears to be a promising skincare and cosmetic ingredients for anti-photoaging agents. However, treatments with laminarin at high concentration have decreased the metabolic activity in dermal fibroblasts and keratinocytes cells [
89]. Therefore, in order to be applied in skincare and cosmetics, further study on laminarin solubility, efficacy evaluation, penetration capacity, half-life time in blood, and bioavailability of laminarin needs to be carried out.
3.5. Phlorotannins
Polyphenolic compounds are a class of secondary metabolites which are categorized into several classes according to the number of phenol rings and structural elements that bind them together [
43]. Phlorotannins are class of polyphenol compounds found exclusively in brown seaweeds and synthesized via acetate–malonate pathway (also known as the polyketide pathway) [
99]. Furthermore, they are also known as seaweeds-chemical defense agents. These bioactive compounds protect seaweeds against grazers, important components of seaweeds cell wall and are responsible for the absorption of UVR [
100].
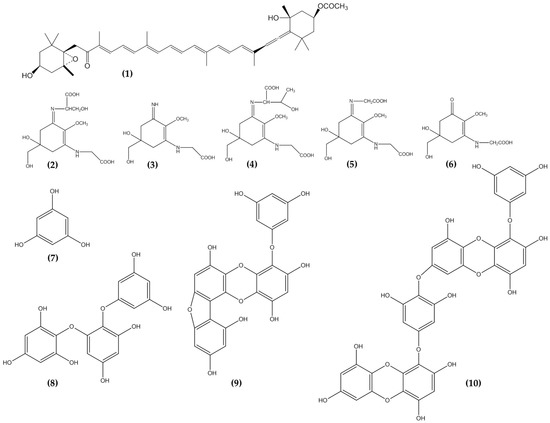
Phlorotannins have been extracted from different brown seaweed resources such as Ecklonia cava, Ecklonia stolonifera, Sargassum thunbergii, Hizikia fusiforme, Endarachne binghamiae, Laminaria sp., and Sargassum piluliferum (). Out of the total brown seaweed species, E. cava was found to contain more total phenol contents [
101]. Compared to other phlorotannins isolated from E. cava, phlorogucinol showed stronger cytoprotective effects in UVB-irradiated HaCaT cells. Currently, the anti-photoaging properties of phloroglucinol are far more explored compared to other phlorotannins. Phloroglucinol showed strong antioxidant activities by inhibiting hydroxyl radical, superoxide radical, and intracellular ROS, and induced the expression of antioxidant enzymes by activating the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2)/ heme oxygenase-1 (HO-1) signaling. Milanovic and colleagues (2020) studied the antioxidant activity of phloroglucinol and 2,4,6-Trihydroxypyridine towards HO· radicals. The study showed that phloroglucinol is a more powerful antioxidant compared to 2,4,6-Trihydroxypyridine [
102]. Furthermore, it was found that the electron-withdrawing effect of nitrogen was stronger than the electron donating effect of the OH groups in the molecule of 2,4,6-Trihydroxypyridine. The structure difference of 2,4,6-Trihydroxypyridine with phloroglucinol is the substitution of nitrogen atom in the aromatic ring of phloroglucinol. Therefore, chemical modifications may affect the scavenging capacity of phloroglucinol. In addition, many studies have showed that the anti-photoaging activity of phlorotannins is strongly related to their radical scavenging activity. The hydroxyl (–OH) group bound to the aromatic ring donates electron and give it to a free radical or other reactive species. This underlies the inhibition of ROS and ROS-mediated damage on macromolecules, which in turn contributes to inhibiting the activation of the signal transduction pathways such as the NF-κB, mitogen-activated protein kinase (MAPK) signaling pathway.
Table 4. Phlorotannin extracted from brown seaweed with potential anti-photoaging activity.
Phlorotannins represent great potency as active anti-photoaging substances by providing multiple actions such as antioxidant, anti-inflammatory, MMP-inhibition, and down-regulation of pro-apoptotic factors. Based on to a certain level of concentration, they do not exert any toxic effect, anticipating its potential use as safe anti-photoaging agents in skin care and cosmetic products. The other biological activity such as anti-microbial activity of phlorotannins shows potency of phlorotannins as natural preservatives in skincare and cosmetic products. Therefore, besides functioning as anti-photoaging agents, they also show great potential to be used as skincare and cosmetic agents with other potential skin benefit effects.
3.6. Mycosporine Like Amino Acids
Mycosporine-like amino acids are LMW, water-soluble molecules that strongly absorb UVA and UVB; generally MW of MAAs are (<400 ~Da) [
120]. These colorless LMW molecules are widely distributed in natures and could be found in many organisms such as phytoplankton, terrestrial lichens, cyanobacteria, coral, cnidarians, sponges, shrimp, sea urchins, starfish, clams, ascidians, and seaweeds [
121]. Differing with photosynthetic pigments, MAAs is invoked to function as a passive shielding substances by dissipating the absorbed radiation energy in the form of harmless heat without generating photochemical reactions [
122]. Their absorption maxima are around 310 to 360 nm depending on the molecular structure [
13,
123]. Based on the structural view, MAAs consists of cyclohexenimine ring conjugated with two amino acid, amino alcohol or amino group substituents [
124].
Mycosporine-like amino acids are demonstrated as one of the strongest naturally occurring UVA-absorbing molecules [
13]. Currently, they have been identified from more than 500 seaweed species [
125,
126]. Furthermore, when compared to other seaweed classes, the red category is an excellent source of MAAs. Sun et al. (2020) stated that in seaweeds, they are mainly distributed in orders Bangiales, Ceramiales, Gigartinales, and Gracilariales. In , information is provided on several MAAs present in seaweeds. Furthermore, during the last five years, a growing number of papers focusing on anti-photoaging properties of MAAs from seaweeds have been observed.
Table 5. Mycosporine like amino acid extracted from different seaweed species.
The anti-photoaging activities of MAAs are not only mediated by their photoprotective activity by absorbing UVR, but also by strong antioxidant, radical scavenging, macromolecule damage-protection, anti-inflammatory, MMP inhibitor, and other potential anti-photoaging activities. Furthermore, the antioxidant activity of seaweeds derived MAAs such as porphyra-334, shinorine, asterina-330, palythine and mycosporine -glycine (Myc-Gly) have been tested in various assays. These include 2,2′-Azinobis-(3-Ethylbenzothiazoline-6-Sulfonic Acid Assay (ABTS+) radical scavenging, β-carotene/ linoleate bleaching method, scavenging capacity of superoxide radicals, Oxygen Radical Absorbance Capacity (ORAC-fluorescein) Assay, ROS scavenging [
127,
132,
133]. In general, MAAs showed strong antioxidant activities. However, the exact mechanisms are still unknown and further investigations need to be carried out on the antioxidant mechanisms of MAAs.
In addition, MAAs derived from seaweed also showed photoprotective activity in HaCaT cells by protecting DNA damage from UVB radiation [
134]. Recently, it was demonstrated that Porphyra-334 and shinorine treatment activated Nrf2/Kelch-like ECH-associated protein 1 (Keap1) pathway. Porphyra-334 and shinorine first dissociated Nrf2 from Keap1. Increased mRNA expression of Nrf2 targeted genes encoding oxidative stress defense proteins prior and post UVR exposure were observed [
135]. Treatment of shinorine and Porphyra-334 in UV irradiated mice was found to increase the expression of endogenous antioxidant (SOD, GSH-Px, CAT), and decrease malondialdehyde expression [
136]. Seaweed-derived MAAs showed antioxidant properties through several functions which include strong UV absorption, protecting macromolecules damage, and antioxidant capacity.
Seaweeds-derived MAAs have also been tested for their anti-inflammatory properties in UV-irradiated HaCaT cells [
137]. Porphyra-334 treatments suppressed COX-2 expression and one of the main cytotoxic mediators participating in the innate response in mammals [
138]. In addition, Shinorine and Porphyra-334 treatment in LPS-stimulated macrophages cells showed potential anti-inflammatory properties. While MAAs treatment significantly suppressed the release of pro-inflammatory mediators which were mediated through NF-κB signaling pathway [
139]. Supporting these results, Poprhyra-334 treatment in UV-irradiated mice also inhibited the activation of NF-κB and MAPK signaling pathways [
140]. Furthermore, many intracellular signaling pathways are involved in inflammatory responses. However, NF-κB and MAPK are amongst the most important signaling molecules involved in inflammatory responses [
141]. Collectively, these reports have showed MAAs as potential anti-inflammatory agents stimulated by UV-irradiation.
Collagen is the major structural protein of the extracellular matrix (ECM) that provides supportive framework to the cell and is responsible for strength, elasticity, and hydration of the skin. [
142] Therefore, collagen and ECM play an important role in skin health, beauty, and aging. Porphyra-334 showed potential anti-photoaging properties by inhibiting MMP-1 and MMP-3 levels. Treatment of Porphyra-334 in human dermal fibroblast cells increase ECM components, such as procollagen, type I collagen, elastin [
132,
143]. Porphyra-334 also showed an inhibition of advanced glycation end products (AGEs) [
143]. The results indicated that treatment with Porphyra-334 maintains the structural integrity of collagen fibers by absorbing ultraviolet radiation. Therefore, Porphyra-334 showed great potential function in preventing skin photoaging.
Among other seaweed-derived MAAs, Porphyra-334 is the most studied MAAs. They have also been reported to down regulate caspase-3 protein expression in UV irradiated HaCaT, suggesting another anti-photoaging properties were also mediated by the down-regulation of pro-apoptotic factors [
134]. Suh et al. (2017) studied the expression profiling of Porphyra-334 modulated genes or microRNA (miRNAs) in response to UV-exposure and their functional networks. It was found that Porphyra-334 regulated Wnt (Wingless/integrase-1; related to UV-repressed genes) and Notch signaling pathways. Furthermore, it is assumed that Porphyra-334 protects cells from UV-induced photoaging through the comprehensive modulation of expression patterns of genes involved in UV-mediated biological processes [
144].
Sunscreen cream containing 0.005% MAAs extracted from
P. umbilicalis (nori) was found to neutralize photodamage caused by UVA radiation as efficiently as cream containing 1% synthetic UVA and 4% UVB filters [
145]. Furthermore, the formulation of Porphyra-334 increased the photoprotective activity of sunscreen formula [
146]. MAAs protects the skin cells by their ability to disperse harmful UV into heat that dissipates into the surroundings without forming reactive photoproducts. The treatment with MAAs was able to inhibit skin wrinkle depth, roughness, and elasticity. This suggests that MAAs are effective and potential anti-photoaging agents. In a recent article, it was found that sunscreen formulated with MAAs showed the same Sun Protecting Factor (SPF) and UVB-Biological Effective Protection Factors (BEPFs) as reference sunscreens but slightly lower UVA-BEPFs [
147].
3.7. Carotenoids
Carotenoids are essential natural pigments along with chlorophylls in photosynthetic organisms, bacteria, and fungi. Furthermore, these tetraterpene pigments are involved in photosynthesis and photoprotection. Carotenoids can be classified into two broad groups, namely carotenes (contain no oxygen) and xanthophylls (oxygenated derivatives of carotenes) [
148]. In 2018, around 850 carotenoids were been found, and the number is still increasing [
149]. Among carotenoids isolated from seaweeds, fucoxanthin is a major xanthophyll with diverse biological functions. These carotenoids represent more than 10% of total carotenoids.
The anti-photoaging function of fucoxanthin has been investigated by many studies (). As a consequence of UVB irradiation, cells face an intense oxidative reaction that gives rise to photodamage and photoaging. Furthermore, fucoxanthin isolated from Korean brown seaweeds
Sargassum siliquastrum showed photoprotective properties in UVB-irradiated human fibroblast. A 24 h pretreatment with fucoxanthin (50–250 μM) were able to reduce oxidative stress via ROS scavenging activity and counteract UVB-induced cell damage in dose-dependent manner [
150]. Furthermore, fucoxanthin also showed remarkable ROS scavenging activity in UVB-irradiated mice and HaCaT and HDF cells [
151,
152,
153]. It showed strong antioxidant activity due to its singlet oxygen quenching (
1O
2) and ROS scavenging effects. From the structural view, fucoxanthin has a unique unusual allenic bond and 5,6-monoepoxide in its molecule which plays an important role in ROS scavenging activity [
22]. In addition, functional groups in the terminal ring of fucoxanthin also have an effect in their antioxidant activity. The electron-rich status of fucoxanthin makes this carotenoid an effective radical scavenger [
154].
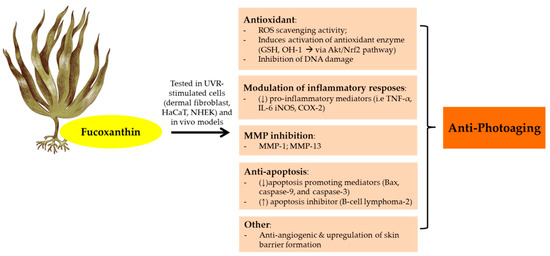
Figure 4. Anti-photoaging activity of fucoxanthin extracted from brown seaweeds. Abbreviations: Reactive oxygen speies (ROS); Human keratinocytes (HaCaT); Normal human epidermal keratinocytes (NHEK);Glutathione (GSH); Protein kinases B (Akt);Nuclear factor erythroid 2–related factor 2 (Nrf2); Matrix metalloproteinase (MMP); Tumor necrosis factor (TNF-α); Interleukin (IL); Inducible nitric oxyde synthase (iNOS); Cyclooxygenase-2 (COX-2); Down-regulated, decreased (↓); Up-regulated, increased (↑).
Inflammatory stimuli could trigger MMP which leads to photoaging, and when UVB reaches our body, keratinocytes which represent the first target act as sentinels, initiate the signaling cascade. These events address the stress and the production of pro-inflammatory factors such as NO, PGE
2, IL-6, IL-1β and TNF-α. Furthermore, Luna et al., (2018) showed that pretreatment of HaCaT cells with fucoxanthin at 50 µM reduced the downstream inflammatory cytokines (TNF-α and IL-6) [
155]. In addition, the synergy effects of fucoxanthin and rosmarinic acid (phenolic ester isolated from
Rosmarinus officinalis L) on UVB-exposed HaCaT have been demonstrated [
156]. A combination of fucoxanthin (5 µM) and rosmarinic acid (5 µM) improved the antioxidant and anti-inflammatory profiles compared to individual compounds. The photo-protective effects of fucoxanthin and rosmarinic acid were mediated by down-regulation of NLR family pyrin domain containing 3 (NRLP3)-inflammasome and upregulation of Nrf2 signaling pathway which further increased the antioxidant gene expression (HO-1).
The levels of structural proteins for the epidermal permeability barrier, including filaggrin (filament aggregating protein) markedly decline in aged skin. UVR has been associated with the level of filaggrin, based on in vitro and in vivo experimental models [
157]. Following UV exposure, filaggrin gene expression was down regulated. Furthermore, treatment with 0.5% fucoxanthin (4 days until day 8) stimulates filaggrin promoter activity and upregulates filaggrin gene expression [
158]. This upregulation of the skin barrier formation by fucoxanthin may contribute to the photoprotective porperties of fucoxanthin. In addition, its treatment protects HaCaT cells from hydrogen peroxide-induced cell death. Fucoxanthin protective actions were mediated by the down-regulation of apoptosis promoting mediators (Bcl-2-associated
X protein (Bax), caspase-9, and caspase-3) and the up-regulation of apoptosis inhibitor (B-cell lymphoma-2 Bcl-2) [
152].
Continuous exposure to UV irradiation induces skin angiogenesis and wrinkle formation [
159]. Furthermore, the topical administration of fucoxanthin (0.001%) prior to UVB radiation in hairless mice showed potential anti-angiogenic effects. It also diminishes epidermal hypertrophy, MMP-13 expression in the epidermis and thiobarbituric acid reactive substances (TBARS) in the skin [
151]. Other studies also showed that fucoxanthin treatment ameliorated UVB irradiation-induced corneal damage and down-regulating Vascular endothelial growth factor (VEGF) expression [
160].
The possibility of administering fucoxanthin topically faces several drawbacks because of the issue of lipophilicity and HMW. Anti-photoaging agents need to diffuse across the stratum corneum and tight junctions to achieve effective permeation. Several vehicle such as hydrogel, cream, and ointment have been tested to achieve the best permeation results with cream showed the most favorable vehicle for fucoxanthin topical administration [
155]. Furthermore, a cream containing fucoxanthin was applied in UVB-irradiated erythema model in hairless mice. It showed photoprotective properties through the down-regulation of COX-2 and iNOS and the up-regulation of HO-1 protein via Nrf-2 pathway. In addition, the effects of fucoxanthin (0.5% in alkyl benzoate or in EtOH) in reconstructed human skin have also been investigated and it was found that its topical applications were safe. Fucoxanthin treatment upon UVB irradiation in reconstructed human skin ameliorated pro-inflammatory mediators (IL-6 and IL-8) [
161]. Collectively, it is believed that fucoxanthin could be a natural adjuvant for preventing photoaging.