To induce broadly protective immune responses by vaccination, various strategies using live attenuated influenza vaccines (LAIVs) and novel vaccine platforms are under investigation. Despite superior cross-protection ability, very little attention has been paid to LAIVs for the development of UIV.
- influenza live attenuated vaccine
- universal vaccine
- antibody
- T cell
1. Introduction
Influenza viruses have posed serious threats on human public health worldwide despite development of effective vaccines and antiviral drugs. Each annual influenza epidemic affects 5−15% of the population and causes 3−5 million cases of hospitalization, claiming 290,000−650,000 lives worldwide [1]. The substantial levels of antigenic diversity and variability of influenza viruses and zoonotic transmission of non-human influenza viruses to humans present persistent possibilities of human infections with novel influenza viruses to which most population has little or no preexisting immunity. Currently-licensed seasonal influenza vaccines have proven effective against well-matched strains. There are three types of influenza vaccines clinically used for humans; inactivated influenza vaccines (IIVs), live attenuated influenza vaccines (LAIVs), and recombinant hemagglutinin (HA) subunit vaccines. IIVs and HA subunit vaccines primarily induce HA-specific neutralizing antibodies that inhibit the binding of viral HAs to cellular receptor sialic acids, thereby preventing viral entry into cells. HA inhibitory antibodies, however, provide very narrow strain-specific protection since the HA head region harboring the receptor binding site is highly variable among influenza viruses. Therefore, antigenic change in the HA head region by antigenic drift often leads to viral escape from the antibodies. This is why the HA and NA of seasonal influenza vaccines are updated almost annually to match newly-circulating strains. In addition, pandemic outbreaks often result from genetic reassortment between influenza viruses from different species, which is unpredictable when a pandemic will occur. In the case of a pandemic, most of the population remains vulnerable to infection with the novel pandemic strain until a well-matched vaccine becomes available.
Extensive efforts to develop a universal influenza vaccine (UIV) that provides broad protection against diverse influenza viruses have been made worldwide [2]. Induction of antibodies and cell-mediated immune responses directed to conserved viral antigens is the key to eliciting broad protection. Since the discovery of broadly neutralizing antibodies to the conserved HA stalk region, several strategies have been advanced, such as chimeric HAs and headless HAs. The HA stalk-based approaches have been successful to selectively induce HA stalk antibodies that exhibit broad protection in animal models and are currently under clinical evaluation. In addition to HA stalk-based approaches, a number of rational strategies have been designed to express cross-reactive antigens (such as NA or M2e) or T cell epitopes in multiple vaccine platforms, such as viral vectored vaccine, recombinant protein or peptide vaccines, DNA or RNA vaccines, virus-like particles, and nanoparticles [3]. LAIVs have shown superior protection efficacy not only against homologous influenza viruses but also against mismatched heterologous strains. In particular, cell-mediated immune responses elicited by LAIVs are considered as critical for cross-protection, and other factors, such as mucosal IgA antibodies and non-specific protection, have also been shown to correlate with cross-protection. Despite abundant experimental and clinical evidence for cross-protection, LAIVs have received little attention as target platforms for a UIV, with only a few recent studies demonstrating exceptional cross-protection abilities by LAIVs in animal models. Studies have determined that induction of the multiple correlates of protection are necessary for providing broad and potent cross-protection against both HA group 1 and 2 influenza A viruses [4]. It has been suggested that antibody effector functions, NA antibodies, and mucosal IgA antibodies are important for cross-protection and thus should be included in a UIV.
2. Principles and Cross-Protection of LAIVs
2.1. Cold-Adapted Live Attenuated Influenza Vaccines
Among various types of LAIVs developed so far, cold-adapted LAIVs (CAIVs) are currently licensed for clinical uses in humans. CAIVs are established by serial passages of parental influenza viruses at low temperatures in embryonated chicken eggs or chicken cells, making the virus less replicative at normal and elevated body temperatures. The resulting cold-adapted virus exhibits cold-adapted (ca), temperature-sensitive (ts), and attenuated (att) phenotypes and, thus, can be used as a safe CAIV donor strain. Genetic and phenotypic analysis have revealed that mutations in the polymerase genes and the NP gene are crucial for the expression of the ca, ts, and att phenotypes [5,6,7,8]. A particular CAIV is constructed by genetic reassortment with the six internal cold-adapted donor strains and the two surface genes, HA and NA, originated from a wild-type circulating virus. Five strains of CAIVs are currently licensed for the manufacture of seasonal CAIVs: A/Leningrad/134/17/57 (H2N2), A/Leningrad/134/47/57 (H2N2), A/Ann Arbor/6/60 (H2N2), B/USSR/60/69, and B/Ann Arbor/1/66 strains. These CAIVs have served as donor strains for the development of seasonal influenza A and B virus vaccines and also other pandemic or prepandemic vaccines [9]. While CAIVs based on A/Leningrad/134/17 and B/USSR/60/69 strains are used in people aged three or older, CAIVs based on A/Ann Arbor/6/60 and B/Ann Arbor/1/66 strains are licensed for use for people aged 2–49 years. Other independent CAIVs, A/X-31 (H3N2) and B/Lee/40 ca, were generated in South Korea but have not entered clinical development yet. CAIVs are delivered intranasally, via the same route of entry as wild-type influenza viruses and, thus, are expected to induce similar immune responses to those by natural infection. CAIVs only weakly replicate in the upper respiratory tracts but rarely in the lower respiratory tracts at normal or increased body temperatures. Replication of CAIVs induce antibody responses and cell-mediated immune responses both systemically and locally, generating multiple immune arms for protection (Figure 1). The immune responses elicited by a CAIV include local mucosal IgA antibodies, serum hemagglutinin-inhibitory (HI) and neutralizing antibodies, and T cell immunity [10]. While it is generally believed that these multiple factors cooperatively contribute to superior protection as compared to IIV, particular functional roles of individual factors have not been clearly defined yet.
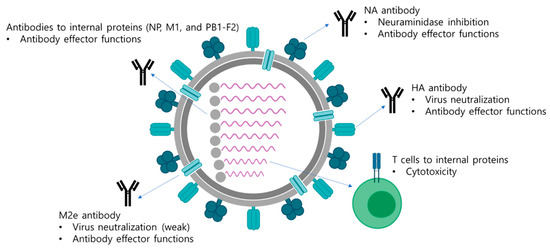
Figure 1. Multifaceted immune responses induced by a LAIV. A LAIV mimics a natural infection and induces multifaceted immune responses including antibody responses and T cell responses. Antibodies directed to surface antigens, HA, NA, and M2e neutralize the virus and also mediate antibody effector functions, such as ADCC, ADCP, and CDC, to eliminate virus-infected cells. The internal viral proteins are also target antigens for generation of antibodies with effector functions. Whether the effector functions of antibodies directed to the internal proteins have protective roles has not been clearly elucidated yet. In addition, cytotoxic T cells are directed to the internal viral proteins, which mediate the elimination of virus-infected cells via direct contact of cytokine production.
2.2. Other Types of LAIVs
Reverse genetics technology has made it possible to introduce targeted mutations into the genome of influenza viruses for generation of a number of genetically engineered influenza viruses. This resulted in various attenuation strategies for the development of novel types of LAIVs (Table 1). Influenza NS1 is a non-structural protein that acts as an interferon antagonist, and deletion or truncation of the NS1 has been shown to result in significant attenuation of viral replication. Studies have shown that monovalent or multivalent LAIVs expressing truncated or mutated NS1 are highly attenuated, immunogenic, and provide robust protection against homologous as well as heterologous influenza virus infections in animals and humans [11]. Of note, a recent study showed that del-NS1 H1N1 LAIV provided broad protection against H1N1, H5N1, and H7N9 challenges in mice through cross-reactive T cell responses even in the absence of neutralizing antibodies [12]. Furthermore, introduction of caspase recognition sites into the NP and NS1 proteins of an influenza virus resulted in caspase-dependent cleavage of the proteins in virus-infected cells, leading to significant attenuation of the viruses [13]. The mutant viruses were highly attenuated and immunogenic in mice, providing strong protection against homologous challenge. Modification of HA cleavage site into elastase recognition motif resulted in the generation of an attenuated influenza virus that could replicate only in the presence of elastase in cell culture [14]. Subsequent follow-up studies examined the potential of the elastase-dependent influenza viruses to serve as novel LAIVs in animal models [15,16]. A double-attenuated LAIV with elastase-susceptible HA cleavage site and shortened NS1 protein demonstrated increased safety but was still immunogenic in a swine model [15]. Similar strategy could be extended to LAIV against influenza B virus [16]. Introduction of miRNA targeting sequences into NP gene resulted in viral gene silencing and species-specific attenuation of the viruses [17]. miR-21- or miR-192 targeted influenza viruses were highly attenuated in susceptible cells and mice but provided robust homologous and heterologous protection in mice [18,19]. M2-deficient influenza virus infected cells only a single round but could induce robust antibody responses and T cell responses providing effective heterosubtypic protection in mice and ferrets [20,21]. Additionally, genetic engineering of splicing elements in M gene and NS gene and codon deoptimization of NS gene also attenuated influenza viruses, providing additional options for developing LAIVs [22,23]. It should also be noted that over-attenuation of the virus may limit vaccine productivity and also the immunogenicity of LAIVs. Extensive evaluation for the productivity, safety, immunogenicity, and potential risk of reversion into a virulent strain is needed for further development into clinically-relevant LAIV options.
Table 1. Strategies for construction of LAIVs.
| Strategy | Mechanism of Attenuation |
|---|---|
| Cold-adaptation | Genetic mutations accumulated during cold-adaptation result in decreased viral replication at body temperature. |
| Deletion or truncation of NS1 | The lack of interferon antagonist NS1 protein results in decreased viral replication in infected cells. |
| Deletion of M2 ion channel | M2-deficient influenza viruses replicates only in cells expressing M2 proteins but are highly restricted in normal cells. |
| Caspase-dependent cleavage of viral proteins | Cleavage of viral proteins by caspases activated during apoptosis of infected cells results in decreased viral replication. |
| Modification of HA cleavage site | The mutant viruses carrying elastase cleavage site in HA undergo restricted replication because of the absence of appropriate protease. |
| miRNA-mediated gene silencing | The viral genes carrying miRNA-targeted region are degraded in infected cells. |
| Codon deoptimization | Codon deoptimization results in downregulation of viral protein synthesis in infected cells. |
| Engineering of splicing elements | Modification of splicing elements in viral genes results in decreased production of the proteins in infected cells. |
2.3. Cross-Protection by LAIVs
It is well-documented that natural infection with an influenza virus induces broadly reactive T cell responses that provide cross-protection not only against homologous but also against drifted strains and even antigenically distant viruses with different subtypes [24]. The same mechanisms may hold true for cross-protection elicited by LAIVs, although the magnitude of T cell responses can be milder than natural infection due to highly restricted replication of the attenuated vaccines. In the literature, there is a large body of experimental evidence for cross-protection by LAIVs [10]. LAIVs have demonstrated considerably varying levels of protection window in different studies from subtype-specific to pan-influenza A protection. A limited number of controlled human studies showed cross-protection of CAIVs against drifted H1N1 or H3N2 strains that were not contained in the vaccines [25,26]. In animal studies, LAIVs have been shown to provide heterosubtypic and even heterotypic protection [10]. A/Ann Arbor/6/60 ca (H2N2) donor strain and A/Ann Arbor/6/60 ca-based 2009 pandemic H1N1 vaccine provided heterosubtypic cross-protection against H5N1 viruses in mice and ferrets [27,28]. A/X-31 ca (H3N2) donor strain protected mice from H1N1 challenge [29], and A/X-31 ca-based 2009 pandemic H1N1 vaccine provided protection against H1N1, H3N2, and H5N2 viruses in mice, showing broad protection against both HA group 1 and 2 influenza A viruses [30]. Of note, the A/X-31 ca donor strain provided heterotypic protection against influenza B virus in a mouse model, although the protection was shown to be mediated by innate immunity not by specific antibody responses. While cross-protection by LAIVs has been extensively examined in animal models, the potency and the breadth of cross-protection in humans remain poorly understood due to several limitations. In humans, controlled challenges with antigenically distant viruses to a vaccine strain is hardly possible due to potential risk of lethal infection even in vaccinated groups. Moreover, in vitro measurements of antibody or T cell-mediated protection against heterologous viruses cannot be interpreted as genuine protection efficacy in vivo since in vitro assays reflect protection mediated by only a part of whole immune correlates induced by vaccination. As discussed below, cross-protection by LAIVs can be ascribed to multiple correlates in addition to T cell responses (Table 2). Better understanding on protection mechanisms by individual immune correlates against influenza viruses and deeper characterization of immune responses induced by LAIVs present the first step towards a development of a truly UIV based on a LAIV.
Table 2. Cross-protective immune responses elicited by LAIVs.
| Immune Responses | Mechanism of Attenuation |
|---|---|
| HA stalk antibodies | Viral neutralization Antibody effector functions |
| NA antibodies | Neuraminidase inhibition Antibody effector functions |
| M2e antibodies | Viral neutralization Antibody effector functions |
| Antibodies to internal proteins | Antibody effector functions |
| T cell response | Cytotoxicity to virus-infected cells |
| Mucosal immunity | Viral neutralization Non-neutralizing activity |
| Innate immunity | Non-specific effects |
This entry is adapted from the peer-reviewed paper 10.3390/vaccines9040353
