Pleomorphic hyalinizing angiectatic tumor (PHAT) is a very rare entity of soft tissue considered a “neoplasm of uncertain behaviour of connective or other soft tissue” by the World Health Organization (2020).
- soft tissue
- PHAT
- immunohistochemistry
1. Introduction
Pleomorphic hyalinizing angiectatic tumor (PHAT) is a very rare entity of soft tissue, first described in 1996 by Smith et al. in a series of 14 cases as a “low-grade sarcoma of uncertain lineage” [1]. In “Soft Tissue and Bone Tumours” by the World Health Organization 2020, PHAT is considered a “neoplasm of uncertain behavior of connective or other soft tissue” [2] and is defined as “very rare” with, to our knowledge, about 100 cases reported in the international literature. PHAT affects patients from 10 to 79 years of age, with slight female predilection [3,4], and it develops in subcutaneous tissue of the lower extremities, more frequently in the region of the ankle and foot [4], and rarely as a deep-seated soft tissue mass in locations as perineum, buttock, arms, head and neck, and viscera [5]. Although inconsistent cytogenetic data have been reported [2] in PHAT so far, there are potential morphological and genetic overlaps with hemosiderotic fibrolipomatous tumor (HFLT) and myxoinflammatory fibroblastic sarcoma (MIFS) [2,4]. However, the link between these cancers remains controversial and not fully understood [2,3].
2. Case Report
A 48-year-old woman was referred to the U.O.C. of Plastic Surgery for a volumetric increase in a mass at the level of the right thigh, present for about 15 years and which in recent months had begun to cause functional discomfort. An echography (US) had confirmed the presence of an intensely vascularized subcutaneous lesion and in agreement with the patient it was decided to opt for the surgical option. The patient underwent a wide surgical excision with histologically confirmed margins free from neoplasm.
The sample had therefore been sent to our laboratory and it appeared as a lesion of 6 × 5.5 × 5 cm, with a multiple chambered and collated appearance when cut and a greyish color (Figure 1).
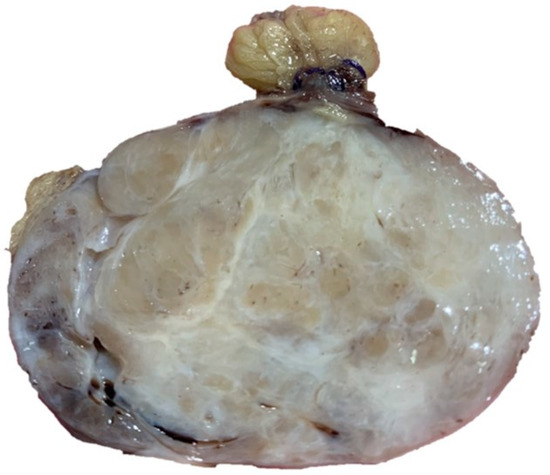
Figure 1. Collated appearance when cut and a greyish color.
After sampling, processing, inclusion in paraffin and microtome cutting, 5-micron thick sections were prepared and stained with routine staining (Hematoxylin-Eosin) and other sections were prepared by immunostaining with anti-CD34, Desmin, Vimentin, Actin Smooth muscle, Ki67 (MIB1), and S-100 protein.
Microscopically, the lesion was composted of clusters of variably sized, thin walled, ectatic blood vessels scattered and surrounded by a thick rim of amorphous eosinophilic material, with fibrosis (Figure 2A,B). There were also organizing thrombi within blood vessels and the tumour cells were arranged in fascicles or, less frequently, sheets with spindle morphology (Figure 2C), sometimes hemosiderin pigment, and nuclear pseudo inclusions. At the periphery, the lesion showed a pseudo infiltrative pattern of growth. There were not cytological atypia and mitotic figures. From the immunohistochemical point of view, we found that the lesion was positive for CD34 (Figure 2D) and Vimentin, while it was negative for Desmin, S-100 protein and smooth muscle actin. The fraction of neoplastic proliferation (valued by KI67) was <5%.
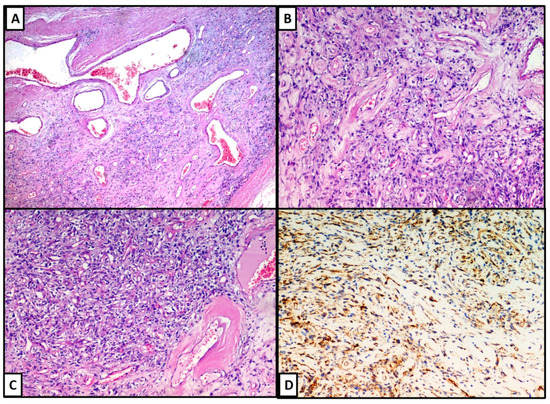
Figure 2. (A–C): The neoplasia was composted of clusters of variably sized, thin walled, ectatic blood vessels scattered and surrounded by a thick rim of amorphous eosinophilic material, with fibrosis phenomena. The lesion was positive for CD34 (D).
After a second opinion consultation with a soft tissue expert pathologist, PHAT diagnosis was placed. At the follow-ups of 6 months and 12 months, the patient showed no local recurrence.
3. Discussion
Pleomorphic hyalinizing angiectatic tumors was firstly described in 1996 by Smith et al. [1] and, to date, about 100 cases have been reported in literature [6]. Table 1 details authors, year of publication of the case series/case report, gender, age, and location of the PHAT.
Table 1. Case of PHAT reported in International Literature.
| Author | Year | Number of pts | Gender | Average Age | Localization |
|---|---|---|---|---|---|
| Silverman, J.S. [7] | 1997 | 1 | F | 59 | Right foot |
| Fukunaga, M. [6] | 1997 | 1 | M | 58 | Axils |
| Gallo, C. [8] | 1997 | 1 | F | 80 | Right popliteal fossa |
| Groisman, G.M. [9] | 2000 | 2 | 2 F | 43 | Lower extremities |
| Matsumoto, K. [10] | 2002 | 1 | F | 83 | Left thigh |
| Iwamoto, C.A. [11] | 2003 | 1 | F | 49 | Axils |
| Folpe, A. [12] | 2004 | 40 | 22 F; 18 M | 53 | ankle/foot (n 15), leg (n 10), thigh (n 6), other (n 9) |
| Fujiwara, M. [13] | 2004 | 1 | M | 69 | Back |
| Lee, J.C. [14] | 2005 | 1 | M | 63 | Right breast |
| Luzar, B. [15] | 2006 | 1 | F | 47 | Ankle region |
| Capovilla, M. [16] | 2006 | 1 | M | 66 | Right buttock |
| El-Tal, A.E. [17] | 2006 | 1 | F | 60 | Right foot |
| Kazakov, D.V. [18] | 2007 | 1 | F | 76 | Axilla |
| Ke, Q. [19] | 2007 | 9 | 5 F; 4 M | 46 | Lower extremities (n 2), inguinal (n 2), waist (n 1), forearm (n 1), buttock (n 1), foot (n 1), chest wall (n 1) |
| Jaggon, J.R. [20] | 2007 | 1 | F | 77 | Left loin |
| Tallarigo, F. [21] | 2009 | 1 | M | 75 | Breast |
| Peng, H.C. [5] | 2010 | 1 | M | 49 | Right buttock |
| Cimino-Matthews, A. [22] | 2010 | 1 | M | 46 | Right calf |
| Parameshwarappa, S. [23] | 2010 | 1 | F | 65 | Upper limb |
| Illueca, C. [24] | 2012 | 2 | 2 F | 51 | Back and eyelid |
| Choong, M.Y. [4] | 2012 | 1 | M | 53 | Inguinal area |
| Subhawong, T.K. [25] | 2012 | 3 | 2 F; 1 M | 51 | Lower extremities (n 2), upper extremity (n 1) |
| Wei, S. [26] | 2012 | 1 | F | 37 | Back |
| Idrees, M.T. [27] | 2012 | 1 | F | 72 | Renal hilum |
| Rekhi, B. [28] | 2013 | 1 | F | 63 | Lower leg |
| Kuang, P. [29] | 2013 | 1 | F | 35 | Neck |
| Changchien, Y.C. [3] | 2014 | 1 | M | 76 | Upper arm |
| Felton, S.J. [30] | 2015 | 1 | M | 61 | Back |
| Morency, E. [31] | 2015 | 1 | F | 55 | Dorsum of foot |
| Brazio, P.S. [32] | 2016 | 1 | F | 22 | Forearm |
| Kane, P.M. [33] | 2016 | 1 | M | 35 | Hand |
| Chu, Z.G. [34] | 2017 | 1 | F | 26 | Retroperitoneal |
| Chalmeti, A. [35] | 2017 | 1 | M | 50 | Left calf region |
| Scalici Gesolfo, C. [36] | 2017 | 1 | F | 61 | Kidney |
| Jaramillo, C.J. [37] | 2018 | 2 | 2 M | 50, 72 | Right buttock (n 2) |
| Szep, Z. [38] | 2019 | 1 | M | 63 | Left crura |
| Balasubiramaniyan, V. [39] | 2019 | 1 | F | 30 | Mesorectum |
| Kökoğlu, K. [40] | 2020 | 1 | F | 33 | Oral cavity |
The mean age at diagnosis was 54.5 ± 17.1 (range 10–89). Of the patients, 61% (60/102) were female. The most common location was the lower extremity in 77.3% (78/102) of the cases, but other localizations were possible such as perineum, buttock, arms, head and neck, and viscera [5]. The average dimensions were around 5.3 cm, with an exceptional case of 26.3 cm in male breast reported by Lee et al. in 2005 [16]. Interestingly, Wei et al. [26] and Chalmeti et al. [35] reported two cases of PHAT respectively at the level of the renal hilum and within the kidney itself, emphasizing the potential problem of confusing this entity with other neoplasms of kidney. Our data completely agrees with Rush et al. [41], who in a recent article in 2018 conducted a detailed review of the literature more focused on the surgical-oncological implications related to PHAT. The best therapeutic strategist is represented by large surgery with free margins [26,41]. The recurrence rate is around 30–40% of cases [2,26,41] when it is not possible to be surgically radical, and local relapses have been described [41], although distant metastases have never been reported [2,41]. Local radiotherapy has also been shown to reduce the rate of local recurrence [26,28,41].
In most of cases it has a lobulated appearance, with a gray to light brown cut surface. It is never encapsulated, and many of these lesions have diffusely infiltrative edges, although well-defined margins [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Sometimes, PHAT can have a prominent cystic component [16,17,18,19]. From a histological point of view, PHAT is composed of clusters of ectatic blood vessels, surrounded by a thick rim of amorphous eosinophilic material, often with associated fibrosis. Immunohistochemically, PHAT is strongly positive for CD34, sometimes CD99 and negative for S-100 protein, Actin, Desmin, Cytokeratin, CD31, Factor VIII and Epithelial membrane antigen (EMA). Groisman et al. [9] reported immunoreactivity for vascular endothelial growth factor (VEGF), but this marker was negative in our case.
The morphological features of PHAT raised a wide range of differential diagnosis such as neurofibroma, schwannoma with ancient aspects, and undifferentiated pleomorphic sarcoma [7,11]. Although neurofibroma may show CD34 positivity, this entity is positive for S-100 protein and EMA, and the absence of cluster ectatic hyalinized vessel allows for a correct differential diagnosis. Unlike the ancient schwannoma, PHAT is encapsulated and it lacks Antoni A and B zones, as well as being negative for S-100 protein [5,6,7]. Finally, low mitotic count, lack of CD34 expression, tumor necrosis and intranuclear cytoplasmic pseudoinclusion can immediately exclude undifferentiated pleomorphic sarcoma [6,11,12,13,14].
This entry is adapted from the peer-reviewed paper 10.3390/dermatopathology8020015
