Isolated limb infusion (ILI) and hyperthermic isolated limb perfusion (HILP) are used to treat melanoma in-transit metastases and unresectable sarcoma confined to the limb utilizing the effect of high-dose loco-regional chemotherapy, normally melphalan +/- actinomycin-D. Both procedures are able to provide high response rates in patients with numerous or bulky lesions in whom other loco-regional treatments are less effective. In comparison to systemic therapies, on the other hand, ILI and HILP result less in severe systemic side-effects. Although in principle ILI and HILP are similar procedures, ILI is technically simpler to perform and takes advantage of the hypoxic and acidotic environment in the isolated limb, potentiating anti-tumour activity of the cytotoxic agents.
- Isolated limb infusion
- melanoma
- sarcoma
1. Principles of Isolated Limb Infusion
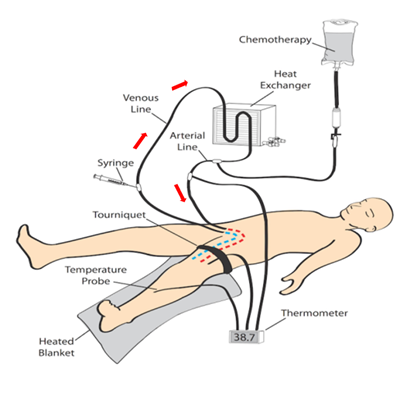
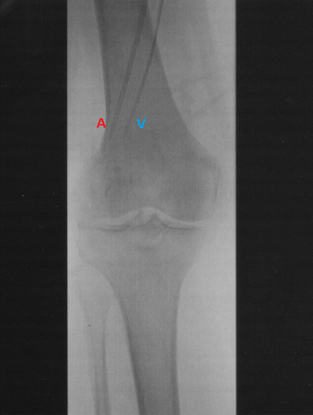
ILI was developed and implemented at the Melanoma Institute Australia (MIA, then called Sydney Melanoma Unit) in the 1990's as a simplified, minimally invasive alternative to HILP [1]. Today, ILI is used at multiple tertiary melanoma referral centres around the world [2][3]. Instead of open surgical cannulation as performed in HILP, percutaneously placed arterial and venous catheters are used to administer the cytotoxic agents, normally melphalan in combination with actinomycin-D, into the affected limb (Figures 1 and 2) [4]. The catheters are placed in the disease bearing limb using the Seldinger technique. The catheter tips are positioned just above the elbow or knee joint with tissues above the tips being perfused in a retrograde fashion via collateral vessels. The limb is then isolated from the rest of the body by placement of a tourniquet at the level of groin or axilla. During ILI, the infusate is not oxygenated, resulting in hypoxia and acidosis of the limb. Prior studies have shown that this environment enhances anti-tumour efficacy of the cytotoxic agents and may be advantageous to increase response rates [5][6]. Moreover, the low flow and pressure in the isolated limb (1000–1500 mL during ILI, in HILP up to 10 times higher) allow for increased drug exposure while avoiding systemic toxicity because of minimal to no leakage of the cytotoxic infusate to the systemic circulation [7]. These measures, and the mild hyperthermia, improve the take up of the cytotoxic agents, augmenting tumour response.
Figure 1. Schematic illustration of the isolated limb infusion circuit for a lower limb procedure (red arrows indicate direction of flow of the infusate).
Figure 2. Angiogram of the arterial (A) and venous (V) catheters positioned in a lower limb with the tips reaching into the mid-popliteal vessels just proximal to the knee.
2. Toxicity Following Isolated Limb Infusion
To report limb toxicity, the Wieberdink toxicity scale, historically used for HILP, has shown to be also applicable after ILI [8]. Although limb toxicity after ILI is at the higher end of the spectrum of that reported for HILP, long-term morbidity is less frequently observed and less severe [9][10]. The majority of patients have no visible toxicity after ILI (grade I toxicity in 33%) or develop slight erythema and/or edema (grade II toxicity in 46%). Considerable erythema and/or edema with blistering (grade III toxicity) is seen in around 19% of the patients. Finally, fasciotomies after ILI, due to threatened or actual severe limb toxicity (grade IV toxicity), are only required in a small number of patients and from all reported series, only one patient has required an amputation due to toxicity (grade V limb toxicity).
No relationship has been identified between increased limb toxicity and complete response (CR) to ILI, duration of response or overall survival (OS), but a relationship was observed between limb toxicity and overall response, which likely shows the challenging balance between maximum efficacy while keeping toxicity low [10][11].
Various pharmacokinetic variables can predict limb toxicity after ILI. A high melphalan dosage, for instance, has been identified as a predictor of increased limb toxicity, and patients with longer ischemia times experience more severe limb toxicity [10][11]. Since uptake of melphalan is higher in muscles as opposed to fat, the skin and subcutaneous tissues of overweight patients are exposed to a relatively higher dose of melphalan [31]. In view of this, attempts have been made to lower the melphalan dose according to the patient’s ideal body weight (IBW), to reduce toxicity without jeopardising efficacy of the treatment [12][13].
After ILI, serious systemic side-effects, such as bone marrow depression or end-organ failure, are rarely seen, while most patients experience only mild postoperative nausea and vomiting, which normally resolves quickly with conservative management [10][11]. The reason for the low number of patients with systemic toxicity can mainly be attributed to effective isolation of the treated limb by placing a tourniquet around its base, while there is already low influx of chemotherapy into the systemic circulation due to the low flow and pressure in the isolated limb circuit.
3. Clinical Response Following Isolated Limb Infusion
In most patients, cutaneous tumour deposits show signs of involution within 7–14 days following ILI. Sometimes, however, it can take several weeks before tumour deposits decrease appreciably in size.
The main goal of ILI is to achieve a CR, as this is associated with an increased OS [14]. Moreover, an overall response (complete and partial response combined) increases OS and improves the quality of life markedly [15]. After ILI, overall response rates of 64 to 73% have been reported, with a median OS of 38 to 101 months [9]. The reported overall response rates after HILP are somewhat higher, but it must be borne in mind that ILI is often performed in older and fragile patients with more comorbidities [16].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9020163
References
- Thompson, J.F.; Waugh, R.C.; Saw, R.P.M.; Kam, P.C.A. Isolated limb infusion with melphalan for recurrent limb melanoma: A simple alternative to isolated limb perfusion. Reg. Cancer Treat. 1994, 7, 188–192.
- Miura, J.T.; Kroon, H.M.; Beasley, G.M.; Mullen, D.; Farrow, N.E.; Mosca, P.J.; Lowe, M.C.; Farley, C.R.; Kim, Y.; Naqvi, S.M.H.; et al. Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: An international multicenter analysis. Ann. Surg. Oncol. 2019, 26, 2486–2494.
- Coventry, B.J.; Kroon, H.M.; Giles, M.H.; Henderson, M.; Speakman, D.; Wall, M.; Barbour, A.P.; Serpell, J.; Paddle, P.; Coventry, A.G.; et al. Australian multi-center experience outside of the Sydney Melanoma Unit of isolated limb infusion chemotherapy for melanoma. J. Surg. Oncol. 2014, 109, 780–785.
- Kroon, H.M.; Huismans, A.; Waugh, R.C.; Kam, P.C.; Thompson, J.F. Isolated limb infusion: Technical aspects. J. Surg. Oncol. 2013, 109, 352–356
- Kroon, H.M.; Thompson, J.F. Isolated limb infusion: A review. J. Surg. Oncol. 2009, 100, 169–177.
- Zaffaroni, N.; Villa, R.; Orlandi, L.; Vaglini, M.; Silvesrini, R. Effect of hyperthermia on the formation and removal of DNA interstand cross-links induced by melphalan in primary cultures of human malignant melanoma. Int. J. Hyperth. 1992, 8, 341–349.
- Padussis, J.C.; Steerman, S.N.; Tyler, U.S.; Mosca, P.J. Pharmacokinetics & drug resistance of melphalan in regional chemotherapy: ILP versus ILI. Int. J. Hyperth. 2008, 24, 239–249.
- Wieberdink, J.; Benckhuysen, C.; Braat, R.; Van Slooten, E.; Olthuis, G. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur. J. Cancer Clin. Oncol. 1982, 18, 905–910.
- Kroon, H.M.; Huismans, A.M.; Kam, P.C.; Thompson, J.F. Isolated limb infusion with melphalan and actinomycin D for melanoma: A systematic review. J. Surg. Oncol. 2014, 109, 348–351.
- Kenyon-Smith, T.J.; Kroon, H.M.; Miura, J.T.; Teras, J.; Beasley, G.M.; Mullen, D.; Farrow, N.E.; Mosca, P.J.; Lowe, M.C.; Farley, C.R.; et al. Factors predicting toxicity and response following isolated limb infusion for melanoma: An international multi-centre study. Eur. J. Surg. Oncol. 2020, 46, 2140–2146.
- Kroon, H.M.; Moncrieff, M.; Kam, P.C.A.; Thompson, J.F. Factors predictive of acute regional toxicity after isolated limb infusion with melphalan and actinomycin D in melanoma patients. Ann. Surg. Oncol. 2009, 16, 1184–1192.
- Huismans, A.M.; Kroon, H.M.; Haydu, L.E.; Kam, P.C.A.; Thompson, J.F. Is melphalan dose adjustment according to ideal body weight useful in isolated limb infusion for melanoma? Ann. Surg. Oncol. 2012, 19, 3050–3056.
- McMahon, N.; Cheng, T.Y.; Beasley, G.M.; Spasojevic, I.; Petros, W.; Augustine, C.K.; Zipfel, P.; Padussis, J.C.; Sanders, G.; Tyler, D.S. Optimizing melphalan pharmacokinetics in regional melanoma therapy: Does correcting for ideal body weight alter regional response or toxicity? Ann. Surg. Oncol. 2009, 16, 953–961.
- Miura, J.T.; Kroon, H.M.; Beasley, G.M.; Mullen, D.; Farrow, N.E.; Mosca, P.J.; Lowe, M.C.; Farley, C.R.; Kim, Y.; Naqvi, S.M.H.; et al. Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: An international multicenter analysis. Ann. Surg. Oncol. 2019, 26, 2486–2494.
- Jiang, B.S.; Speicher, P.J.; Thomas, S.; Mosca, P.J.; Abernethy, A.P.; Tyler, D.S. Quality of life after isolated limb infusion for in-transit melanoma of the extremity. Ann. Surg. Oncol. 2015, 22, 1694–1700.
- Teras, J.; Kroon, H.M.; Miura, J.T.; Kenyon-Smith, T.; Beasley, G.M.; Mullen, D.; Farrow, N.E.; Mosca, P.J.; Lowe, M.C.; Farley, C.R.; et al. International multicentre experience of isolated limb infusion for in-transit melanoma metastases in octogenarian and nonagenarian patients. Ann. Surg. Oncol. 2020, 27, 1420–1429.