Endometriosis is a chronic, inflammatory, hormone-dependent disease characterized by histological lesions produced by the presence of endometrial tissue outside the uterine cavity. Despite the fact that an estimated 176 million women are affected worldwide by this gynecological disorder, risk factors that cause endometriosis have not been properly defined and current treatments are not efficient. Although the interaction between diet and human health has been the focus of many studies, little information about the correlation of foods and their bioactive derivates with endometriosis is available. In this framework, Brassica crops have emerged as potential candidates for ameliorating the chronic inflammatory condition of endometriosis, due to their abundant content of health-promoting compounds such as glucosinolates and their hydrolysis products, isothiocyanates.
- aquaporins
- Brassica
- isothiocyanates
- indoles
- endometriosis
- inflammation
1. Introduction
Endometriosis is a frequently diagnosed, incurable, hormone-dependent gynecological disorder characterized by a chronic inflammatory profile and histological lesions generated by the abnormal growth of endometrial-like tissue outside the uterine cavity. These lesions mainly occur within the peritoneal cavity and are engrafted in different locations such as the peritoneum wall, ovaries, colon, and bladder, although it can also develop in distant organs, such as the liver, lung, and brain, among others [1,2]. Endometriosis may appear in diverse forms and locations, and thus it can be classified as peritoneal endometriosis; ovarian endometriosis; deep infiltrating endometriosis (DIE); or extragenital endometriosis [1,2].
It is estimated that approximately 176 million women worldwide are affected by endometriosis, which represents about 10% of women of reproductive age [2]. However, it is suspected that these data could underestimate the real number of affected women, since many of them are accidentally diagnosed after going into surgery for the treatment of other pathologies [1,2]. Women affected by endometriosis may present severe symptoms, which include, among others, chronic pelvic pain, dysmenorrhea, and dyspareunia [1–4]. In this context, 2–4% of women of reproductive age may suffer from sexual dysfunction due to the painful symptoms produced during intercourse [5]. Furthermore, pelvic pain can be incapacitating, especially in cases of DIE [6,7]. Endometriosis causes infertility in approximately 30% of affected women, likely because of the endometriotic lesion scars present in the reproductive organs [1,2,8]. Furthermore, it has been demonstrated that endometriosis is a disease with a high impact on health care costs, being comparable with other chronic diseases such as diabetes [9].
On the other hand, knowledge of the direct influence of diet on human health has grown quickly in recent years [10,11]. However, to date, no conclusive results relating a diet rich in vegetables with endometriosis development or an improvement in its symptoms have been found [12]. In this regard, Parazzini et al. [13] performed a case-control study with 504 women suffering from endometriosis. Food frequency questionnaires revealed that women with a higher intake of vegetables showed a lower endometriosis risk. Nevertheless, another case-control study based on dietary questionnaires found no association between a higher intake of vegetables and a decrease in endometriosis risk [14]. A more recent study showed that women who consumed more than one serving of cruciferous vegetables per day had a 13% higher risk of developing endometriosis compared with those who ingested them once a week or less [15]. However, these data came from retrospective studies based on self-reported questionnaires, while there is little or no information on the effect of a diet rich in Brassica spp. for women affected with endometriosis and even less information about the effect of the bioactive compounds present in cruciferous foods.
2. Brassicaceae and Their Bioactive Compounds in Inflammation
Brassica crops are well known for their high content in health-promoting compounds [94,95]. Specifically, glucosinolates (GLSs) and their bioactive breakdown product, isothiocyanates (ITCs), have shown different types of activity, such as induction of detoxification Phase II enzymes [96] and anti-tumorigenic [97] or anti-inflammatory effects [98]. Glucosinolates are secondary plant metabolites that are mainly present in vegetables from the Brassicaceae family. Their basic structure is a thiohydrozimate-O-sulfonate group linked with a glucose, whose side chain can vary depending on the amino acid from which they are derived [99]. Inside the plant cells, GLSs are stable, but when tissue disruption takes places, for example, chewing or wounding, these biomolecules are hydrolyzed by the enzyme myrosinase (EC 3.2.1.147), producing ITCs [100]. Cooking methods can also affect the content in glucosinolates and its degree of conversion to ITCs. For example, steaming, microwave cooking, and stir frying are processes that reduce the glucosinolate content the least and can even improve its extraction from the food matrix [101]. However, boiling or blanching usually inactivate myrosinase while leaching glucosinolates and breakdown products into the water [102]. A link between chronic inflammatory-related diseases, such as cancer or obesity, and these bioactive ITCs has been reported [10,103]. Furthermore, different ITCs are involved in diverse mechanisms and pathways of inflammatory processes; this analyzed here under the perspective of endometriosis.
2.1. Aliphatic ITCs and Related Metabolites
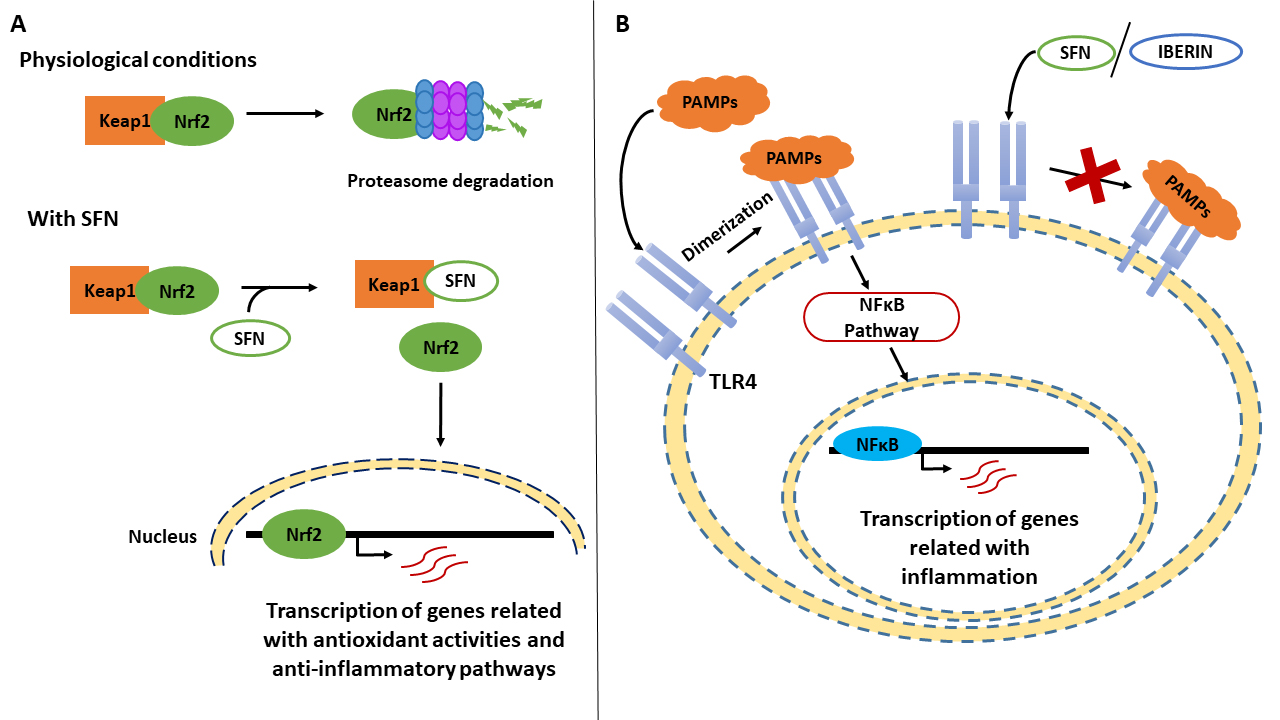
One of the most frequently studied aliphatic ITCs is sulforaphane (SFN), the resulting hydrolysis derivative of glucoraphanin, due to its effects on human health. SFN is widely considered to be a multi-faceted agent due to its role in several cellular pathways—attenuating, reversing, or even blocking different activities in the cellular metabolism [97]. For example, it has been reported that SFN specially induces the activation of Phase II detoxification enzymes, alters cellular signaling pathways, and takes part in the suppression of pro-inflammatory responses [104]. Specifically, SFN has been described as an inductor of the Nrf2 transcription factor (Figure 1A), which is responsible for the transcription of various genes involved in antioxidant activities or anti-inflammatory pathways [105]. The influence of SFN is based on its ability to bind the cysteine residues present in the Nrf2 repressor Keap1. Under normal conditions, Keap1 promotes the degradation of Nrf2 in the proteasome, but when SFN interacts with it, Nrf2 is released and translocated to the nucleus [106].
Figure 1. Effect of Brassicaceae bioactives compounds upon the transcription of genes related with inflammation. (A) In physiological conditions, Keap1 promotes the degradation of Nrf2 by the proteasome. However, when sulforaphane (SFN) interacts with Keap1 by binding its cysteine residues, Nef2 is released and translocated to the nucleus. In this way, Nrf2 up regulates the transcription of genes related with antioxidant activities and anti-inflammatory pathways. (B) In an inflammatory process, Toll-like receptor 4 (TLR4) undergoes dimerization after recognizing pathogen-associated molecular patterns (PAMPs), activating the NFκB pathway and up-regulating genes related with inflammation. When SFN and iberin interact with TLR4, it cannot oligomerizate, and the inflammatory response is inhibited.
Furthermore, SFN is involved in the NFκB pathway, decreasing its ability to bind to target genes related to the inflammatory response, such as pro-inflammatory interleukins, including tumor necrosis factor (TNF)-α [97]. In this signaling cascade, Toll-like receptor (TLR)-4 is the first element, and it is responsible for recognizing pathogen-associated molecular patterns (PAMPs) or intrinsic molecules (Figure 1B), such as fibronectin and heparan sulfate [107,108]. It has been reported that SFN can bind to the cysteine residues in this receptor, suppressing its oligomerization and inhibiting the inflammatory response [109].
Additionally, several pre-clinical studies have demonstrated the efficiency of this ITC in models of chronic inflammatory diseases [110]. For example, Zhao et al. [111] demonstrated that the administration of SFN to a nephropathy murine model reduced the level of reactive oxygen species (ROS) and activated Nrf2 transcription factor. However, little to no information about the effects in models of endometriosis has been found. Recently, Zhou et al. [112] revealed that SFN attenuated endometriosis symptoms in rats by diminishing the levels of TNF-α and IL-6 in peritoneal fluid and plasma. Moreover, SFN seems to decrease the expression of VEGF, affecting the neoangiogenesis of the endometriotic foci. In addition, SFN has been reported to inhibit the growth of ectopic endometrial tissue in sciatic endometriosis rat models, showing a decrease in both the size of lesions and the VEGF level [113]. However, more studies are needed in order to determine the effect of SFN in long-term dietary interventions.
SFN has a structural analog, sulforaphene (SFE), which differs only by having a double bond in the alkyl chain. It is derived from the aliphatic GLS glucoraphenin and can be found mainly in radishes [114]. Although research on SFE has been focused on its anti-carcinogenic and pro-apoptotic effects [115], little is known about its direct effect on inflammatory processes. However, it is known that SFE can lose its double bond, turning into SFN, whose properties are better known [116,117].
Another aliphatic ITC present in Brassica foods, including broccoli sprouts, is erucin [118]. Although an inter-conversion of SFN to erucin and viceversa has been reported in humans after its consumption, the effects of erucin in inflammation are also significant [119]. For example, it has been reported that it decreases the DNA-binding capacity of NFκB, thus reducing the transcription of target genes related to the inflammatory process, including TNF-α, IL-6, COX-2, and inducible nitric oxide synthase (iNOS) [120]. Additionally, glucoiberin is a glucosinolate that is present in products such as cabbage and kale, and its degradation product is iberin [121,122]. As mentioned previously, TLRs play an important role in the induction of the innate immune response, and iberin can prevent the dimerization of TLRs, down-regulating NFκB signaling [107,123].
In oilseed rape and other herbaceous Brassicas, the aliphatic ITC allyl-ITC (AITC) derived from sinigrin does not have sulfur atoms in its side chain, unlike SFN and SFE. The action of AITC in the NFκB pathway when administered to the LPS-activated macrophage RAW 264.7 has been reported to decrease the production of TNF-α, IL-6, and nitric oxide [124]. In addition, in vivo studies performed in rat models of mammary carcinogenesis showed that AITC administration did not provoke a decrease in p65-NFκB expression and, thus, in pro-inflammatory cytokines such as IL-6 [125]. The same group also reported that AITC has a preventive effect on DMBA-induced mammary carcinogenesis by modulating the aryl hydrocarbon receptor (AhR)/Nrf2 signaling pathway [126]. This cytoplasmic receptor and transcription factor has a major role in environmental pollutant detoxification [127]. Nevertheless, recent studies have highlighted the role of AhR as a negative regulator of the immune response [128]. In this regard, it has been reported that AhR-null mice produce higher levels of pro-inflammatory cytokines, such as TNF-α and IL-12 [129].
2.2. Indoles and Related Compounds
The hydrolysis of glucobrassicin, a major indole glucosinolate present in Brassicas, including broccoli or cabbage [130], produces indole-3-carbinol (I3C). I3C can be converted into its dimeric condensation product 3,3-diindolylmethane (3,3-DIM) [131]. Both biomolecules are AhR agonists that decrease the level of IL-1β and increase the rate of the detoxification cascade [132]. In addition, when I3C was administrated to murine models of doxorubicin-induced damage, positive stimulation of the Nrf2 response and down-regulation of p50-NFκB expression was observed [133]. However, when macrophages derived from monocytes of systemic lupus erythematosus patients were treated with I3C, a switch toward a M2 phenotype was described [134]. As mentioned before, M2 macrophages could help with the survival of endometrial cysts [54]. Thus, in the absence of in vivo studies of I3C applied to endometriosis, the specific effect on this disease remains unknown.
On the other hand, 3,3-DIM alone has been reported to inhibit the inflammatory response by also down-regulating the NFκB pathway and decreasing the levels of prostaglandin E2 (PGE2), TNF-α, IL-6, and IL-1β in murine macrophage cell cultures [135], and the oral administration of 3,3-DIM to a model of acute colitis in mice provoked the down-regulation of various types of VEGF and the expression of the VEGF receptor-2 [136]. This function would be beneficial for endometriosis patients to reduce the neoangiogenesis of lesions. In addition, the effects of 3,3-DIM have been also tested in combination with dienogest, a common progestrin that is commonly prescribed to treat pain associated with endometriosis. Women who were administered with this combination for 3 months reported less endometriosis-associated pelvic pain [137]. However, only eight patients finished the study; therefore, studies with a larger number of volunteers are needed to better understand its effects.
Ascorbigen is a biomolecule obtained from glucobrassicin degradation products generated after the enzymatic hydrolysis of I3C in the presence of ascorbic acid that has shown high antioxidant potential [138]. Fermented cabbage extracts enriched in ascorbigen were found to have improved antioxidant capacity and nitric oxide inhibition in RAW 264.7 murine cell cultures [139]. Nonetheless, information about the exact effects of this metabolite in inflammation is very limited since no recent in vivo studies are available.
3. Conclusion and perspectives
To summarize the results presented in this review, macrophages are key immune cells in endometriotic lesions, which are characterized by an anomalous inflammatory environment and cell growth in women with endometriosis.We have presented and discussed a new approach based on the potential role of glucosinolates/isothiocyanates from brassicas as putative natural anti-inflammatory compounds, which could reduce the symptoms and progression of endometriosis, improving the quality of life for a ected women. The potential role of glucosinolates in the development chronic inflammatory diseases should be investigated since there is still little information in this respect.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249397