Nanotechnology has great capability in formulation, reduction of side effects, and enhancing pharmacokinetics of chemotherapeutics by designing stable or long circulating nano-carriers. However, effective drug delivery at the cellular level by means of such carriers is still unsatisfactory. One promising approach is using spatiotemporal drug release by means of nanoparticles with the capacity for content release triggered by internal or external stimuli. Among different stimuli, interests for application of external heat, hyperthermia, is growing. Advanced technology, ease of application and most importantly high level of control over applied heat, and as a result triggered release, and the adjuvant effect of hyperthermia in enhancing therapeutic response of chemotherapeutics, i.e., thermochemotherapy, make hyperthermia a great stimulus for triggered drug release. Therefore, a variety of temperature sensitive nano-carriers, lipid or/and polymeric based, have been fabricated and studied. Importantly, in order to achieve an efficient therapeutic outcome, and taking the advantages of thermochemotherapy into consideration, release characteristics from nano-carriers should fit with applicable clinical thermal setting. Here we introduce and discuss the application of the three most studied temperature sensitive nanoparticles with emphasis on release behavior and its importance regarding applicability and therapeutic potentials.
- hyperthermia
- temperature sensitive nanoparticles
- liposomes
- polymeric nanoparticles
- triggered drug release
1. Introduction
For a chemotherapeutic drug to be effective the concentration reaching the target site (e.g., the tumor) needs to be sufficient enough to eradicate all tumor cells, which is one of the major challenges in chemotherapy. Often the high cytotoxic activity of a therapeutic compound observed in vitro is not matched after administration in animal models or patients. When injected into the bloodstream low-molecular weight chemotherapeutic agents exhibit short plasma half-lives due to distribution throughout the entire body, fast clearance, neutralization, or degradation. While the large volume of distribution and fast clearance rate negatively impact intratumoral drug concentrations and exposure time in the tumor tissue [1], toxic effect on normal tissues or excreting organs simply make dose increase not an option [2].
Encapsulating chemotherapeutic agents into a carrier not only enables administration of poor water soluble drugs [3][4][5] [3–5] or preserves drugs from inactivation and degradation [6][7][8][6–8], it significantly reduces the exposure of normal tissues to chemotherapeutics by decreasing the distribution volume, which is under ideal circumstance a restriction to the blood pool, therefore lessening unwanted side effects on normal tissues. Modifications such as making nano-sized particles and PEGylation, which is the addition of polyethylene glycol to diminish recognition by macrophages, detract recognition of nanoparticle by the reticuloendothelial system (RES, i.e., macrophages) [9], and together with stable association of the encapsulated drug with the vehicle, grant a prolonged circulation of drugs in blood. In principle such modifications aim at taking advantage of pathological features of tumors where permeation into the tumor is enhanced due to fenestrated tumor vasculature and nanoparticles are retained inside tumors due to a lack of a functional lymphatic drainage; a mechanism known as enhanced permeation and retention (EPR) effect [10][11][10,11], and so far EPR-based passive targeting was the central paradigm in cancer nanomedicine. It has been shown that encapsulation of doxorubicin (DXR) in nano-sized PEGylated liposomes resulted in 250-fold reduced clearance rate and 60-fold reduced volume of distribution to 5.9 L compared to 365 L for free DXR when administered in a dose of 50 mg/m2 in humans [12], minifying the main dose-limiting cardiotoxicity associated with DXR treatment [13]. Meanwhile, compared to free DXR, higher accumulation of liposomal DXR in tumor has been achieved [14][15][16][17][14–17]. In this respect several clinically approved nanoparticles have been developed for treatment of cancer. However, the clinical success of these nanodrugs mostly relies on reduced side effect rather than improved antitumor efficacy.
In addition to features of a tumor that are in favor of drug delivery based on EPR, there are several barriers limiting a predictable and effective drug accumulation inside the tumor. Heterogeneous blood supply resulting in hyper-perfused and non-perfused areas prevents a homogenous drug distribution. Additionally, high interstitial fluid pressure and high levels of matrix proteins limit systemic administered drug escaping the blood flow and entering the tumor interstitium. Tumors tend to have an abundance of fibroblasts and infiltrating immune cells re-designed towards a pro-tumoral status. These cells also absorb drugs lowering the availability for malignant cells. Cytotoxic drug levels do not reach all cancer cells causing re-growth of the partially affected tumor. Moreover, heterogeneous drug delivery creates concentration gradients leading to regions with non-cytotoxic drug levels, which may cause drug resistance [18][19][20][21][22][23][24][25][18–25]. Therefore, manipulating the tumor microenvironment to increase perfusion, permeability, and extravasation, or even normalization of tumor vasculatures, were found to enhance drug delivery in tumors [26][27][28][29][26–29]. A dense extracellular matrix in a tumor not only correlates with a high interstitial fluid pressure but also prevents distribution of drugs into deeper regions by decreasing the diffusive or convective movements of drugs inside the tumor interstitial space [30]. This holds true for both free and encapsulated chemotherapeutics but becomes more significant for nanoparticles compared to free drug molecules as a result of a larger size. An effective EPR relies on a homogenous distribution and permeability of the tumor-associated vasculature, which is more than often not the case. Vessel growth in a tumor is very heterogeneous resulting in hyper-vascularized versus non-vascularized areas, mature versus immature (leaky) vessels, and a functional blood flow versus stasis. This results in a very heterogeneous blood supply, nanoparticle delivery, and extravasation [31][32][31,32]. Even extravasated nanoparticles remain predominantly in the perivascular region and only minimal amounts penetrate into the tumor interstitium further [33][34][25,33,34]. Tumor heterogeneity imposes significant limitations on effective delivery of nanoparticles to tumors and is not only observed spatially within a tumor [35][36][35,36], but between individual tumors [37] and is dependent on the size, location, and most importantly growth rate. Compared to model murine tumors, human tumors grow slower making this heterogeneity even more prominent. It seems that passive accumulation of nanoparticles in a tumor via the EPR effect is not that effective in human cancer and worth mentioning that the concept of enhanced permeation i.e., extravasation of nanoparticles through inter-endothelial gaps present in tumor blood vessels, is now under serious debate [38][39][38,39].
Next to challenges with delivery to tumor, cellular availability of drugs encapsulated in nanoparticles is another challenge. For an encapsulated drug to become biologically active it is important that the drug dissociates from the carrier. As stated earlier most of the nanoparticles are built to be stable during circulation in blood or are devised to be unrecognizable by reticuloendothelial system (RES) (coined as stealth), in order to ensure a long blood circulation, which is a prerequisite for tumor accumulation. However, upon entering the tumor interstitium that stability or stealthiness turns against effective delivery of drugs to cells where only free drug molecules can be functional. It could be said that in general content release has been sacrificed in the favor of nanoparticle stability, although release of the drug is essential to achieve a therapeutic response. When PEGylated liposomal doxorubicin (PLD) extravasates into the tumor interstitium minimal DXR release or interactions with cells is observed due to the rigid liposome membrane and presence of Polyethylene glycol molecules. As a result, it remains inside the tumor for a long period of time and DXR delivery to cells mainly takes place upon degradation of liposomes in a slow process over several days [40][31,40]. We showed that even the liposomes taken up by cells remain intact inside the endosomal/lysosomal system for several days [41][31,32,41]. Therefore, accumulation of nanoparticles in a tumor does not imply cellular availability of the content or that targeting of subcellular structures occurs [42].
One approach to enhance cellular delivery is by using ligand-mediated internalization, where nanoparticles decorated with ligands that interact with appropriate receptors on cells are being taken up by endocytosis. However, despite numerous studies none of the preclinically accepted ligand-modified nanocarriers have shown enough clinical benefit to reach the market. Despite ligand-modification of nanoparticles enhances internalization into cells, it has been shown that cellular level of drugs delivered into cells does not necessarily imply the availability of free drug molecules to affect the subcellular targets [41]. Moreover, all problems related to inefficient drug delivery to tumor through nanoparticles still exist since active targeting of tumor cells relies first on passive extravasation to reach the targeted cells. In addition, ligand modification negatively impacts on pharmacokinetics and distribution of nanoparticles; while ligand modification reduces circulation lifetime of nanoparticles, because of augmented clearance rate, which in turn decreases tumor accumulation [43][44][45][43–45], addition of an avidity to nanoparticles results in a prompt interaction with tumor cells soon after extravasation, limiting distribution and penetration of nanoparticles deeper into the tumor [41].
The crucial importance of delivering free drug to tumor cells prompted researchers to exploit pathological features of tumor microenvironment, including reduced pH [46][47][46,47], increased enzymatic [48] or redox [49] activities, and design nanoparticles releasing payload in response to these endogenous stimuli [50][51][52][53][54][50–54]. However, regions with the appropriate climate are heterogeneously distributed throughout the tumor, response to these stimuli is often slow and uncontrollable, and the small difference between normal and malignant tissue necessitates vehicles responsive to a narrow range of differences. Furthermore, delivery of such endogenous trigger-sensitive nanoparticles again relies on passive accumulation. In contrast to endogenous stimuli, exogenous stimuli such as heat, ultrasound [55][56][57][55–57], light [58][59][58,59], and radio frequency electromagnetic fields [60][61][60,61] provide a great spatiotemporal control over triggering the release from nanoparticles. Among these ultrasound [62][63][62,63] and mild hyperthermia [64][65][40,64,65] have an additional advantage to enhance tumor permeability and have been employed to improve tumor accumulation of nanomedicines.
Here we focus on the use of mild hyperthermia (e.g., 42 °C) in the steered delivery and release of coadministered nanoparticles. Thermosensitive nanoparticles can be constructed that are stable in the blood flow (i.e., at temperatures of 37 °C) and release their content when a higher temperature is applied at the tumor site.
2. Hyperthermia and Its Clinical Application
Hyperthermia in treatment of cancer is defined as raising the temperature of tumor locally or systemically by external means and it is now being regarded as the fifth modality of treatment, next to surgery, chemotherapy, radiotherapy, and immune-therapy.
The application of local or systemic hyperthermia for cancer therapy goes back to around 3000 B.C when, for example, the so-called “fire drills” (hot blades and sticks) were being used for the treatment of breast cancer [66]
Application of local hyperthermia can roughly be divided into two strategies. Thermal ablation with temperature above 50 °C and mild hyperthermia using temperatures around 41–45 °C. The high temperature used in ablation irreversibly damages cellular structures, directly killing cells and destroying tissue and matrix components as well as the vasculature, leading to immediate tissue destruction. This is mostly suitable for organ confined tumors, such as hepatocellular carcinomas, however, as the tumor spreads in the surrounding tissue not all cells will be effectively ablated.
Mild hyperthermia can directly and indirectly affect tumor cells. Cells located in regions of the tumor with poor blood flow have higher sensitivity to heat due to the hypoxic and acidic condition and could be selectively killed by application of this type of heat [67][68][69][67–69]. Due to mild hyperthermia cell membrane permeability and tumor perfusion are increased and capability of repairing DNA damages is disturbed [70]. These, on the one hand result in greater drug delivery to tumor and cells, and on the other hand increase the sensitivity of cells to chemotherapeutics and radiation, boosting the effect of chemotherapy and radiotherapy when combined with hyperthermia [71][72][71,72]. In addition, mild hyperthermia can induce or improve antitumor activity [73][74][75][76][73–76]. For example, many of the heat-shock proteins (HSPs) released from heat-stressed cancer cells can activate antigen-presenting cells (APCs) [77][78][79][77–79].
A standard chemotherapy protocol, including doxorubicin, in combination with regional mild hyperthermia has been shown to improve significantly the short-time and long-time survival of soft tissue sarcoma patients as compared to chemotherapy alone [80][81][80,81], most likely because of altered cellular processes, including DNA repair, change in the tumor microenvironment, and anticancer immunity induced by hyperthermia [82].
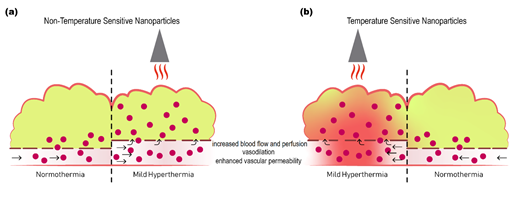
In addition to these effects mild hyperthermia also increases the permeability of tumor vasculature by enlarging pore size in the endothelial lining, facilitating nanoparticle extravasation, which, alongside increased perfusion, improves accumulation of liposomes [83][84][85][83–85] or free drug [86][87][86,87] inside the tumor interstitium (Figure 1). Enlargement of gaps in the endothelial lining is specific for the tumor-associated vasculature and is reversible after approximately 8 h [64].
Delivery of chemotherapeutic agents in a spatiotemporal controlled manner has long been a dilemma in the field of drug delivery. We believe that with good knowledge of the biology, and the unique features of the tumor tissue subjected to heat, and the advanced methods and instruments that enabled precisely controlled application of hyperthermia at desired regions, heat triggered drug release from temperature sensitive nano-devices is the most advanced and promising strategy to fulfill the concept of the magic bullet. However, the rate and magnitude of release from such nanoparticles are crucial with respect to efficient drug delivery. Herein we discuss the advances made with designing three main classes of temperature sensitive nanoparticles.
Figure 1. Schematic representation of the effect of mild hyperthermia on drug delivery to tumors. Delivery of non-temperature sensitive nanoparticles (a), or temperature sensitive nanoparticles (b) to tumor is enhanced by application of mild hyperthermia. Mild hyperthermia increases regional blood flow and perfusion, causes vasodilation, and increases vascular permeability, resulting in increased extravasation and more homogeneous distribution of nanoparticles deeper into the tumor. When combined with temperature sensitive nanoparticles the additional advantage is increased cellular delivery of free drug upon release from the nanoparticles in response to heat.