The purpose of this study was to evaluate the recent literature on the genetic characterization of women affected by endometriosis and to evaluate the influence of polymorphisms of the wingless-type mammalian mouse tumour virus integration site family member 4 (WNT4), vezatin (VEZT), and follicle stimulating hormone beta polypeptide (FSHB) genes, already known to be involved in molecular mechanisms associated with the proliferation and development of endometriotic lesions.
- WNT4
- SNPs
- VEZT
- FSHB
- endometriosis
- genetic
Our analysis of the recent literature shows evidence of many genes possibly implicated in different pathogenetic mechanisms of endometriosis (Figure 1).
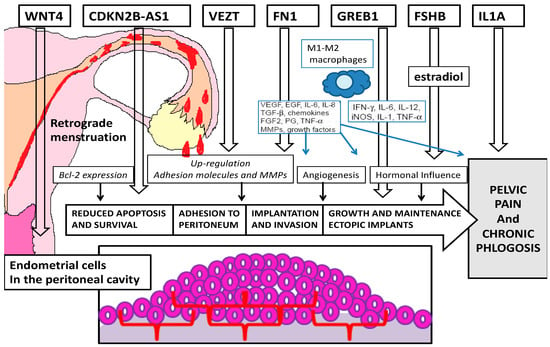
Figure 1. Pathogenetic targets of the most important genes related to endometriosis.
The wingless-type mammalian mouse tumor virus integration site family member 4 (WNT4) gene is positioned on chromosome 1p36.23-p55 and codifies a protein which is essential in developing the female reproductive system [1][2][3][4][5][6]. It critically regulates the appropriate postnatal uterine maturation, as well as ovarian antra follicle growth[2]. The WNT class is an extensive group of secreted glycoproteins, codified through 19 different genes implicated in the WNT signaling pathway[1]. WNT-mediated signal transduction pathways address the specific mobilization of groups of genes which are responsible for managing several cellular responses, including cell growth, differentiation, movement, migration, polarity, cell survival and immune response[1]. Imperfection in WNT4 activity play role in the development of three important organs deriving from the primordial urogenital ridge- the kidneys, adrenal glands and gonads[1]. This may demonstrate the significant position of WNT4 at an early embryological stage of development. The loss of WNT4 in knockout mice determines the total absence of the Mullerian duct and its derivates[3]. Apart from being crucial for epithelial-stromal cell communication in the endometrium, WNT signalling is likely important for endometrial maturation an differentiation and embryonic implantation[7]. An association between endometriosis and markers located in or near WNT4 has been highlighted in a number of extensive studies on gene mapping[8][9]. The expression of WNT4 has also been detected at the level of the peritoneum, leading to the consideration of a possibile metaplastic hypothesis in promoting the transformation of peritoneal cells into endometriosic cells, through pathways with a role in the development of the female genital tract[4]. Pagliardini et al. demonstrated that a single nucleotide polymorphisms (SNP), rs7521902, located 21 kb up/downstream of the WNT4 region, has a susceptibility locus for endometriosis. The functional significance of this SNP in endometriosis remains to be explained[2].
The vezatin (VEZT) gene is located on chromosome 12 locus 12q22; it codifies vexation, a significant element of the cadherin-catenin complex, which plays a crucial role in the formation and sustenance of adherent joints[10][11][12][13]. According to Kussel-Andermann et al., vezatin was found to be a plasma membrane component with a short extracellular domain, a transmembrane domain and an extended intracellular domain. Its intracellular domain connects to myosin VIIA as part of the adherent junctional complex in epithelial cells[11]. Furthermore, several studies on co-immunoprecipitation showed that the system between vezatin and myosin VIIA is able to interact with the system between E-cadherin and catenina, although the specificity of this interaction remains to be determined[11][12]. Also, VEZT is fundamental for implantation; embryos from mice with silenced VEZT cannot develop after the blastocyst stage, because of loss of adhesion between cells[14]. It has been highlighted that VEZT protein is extensively expressed in human endometrium and myometrium. The mRNA expression of adherents junction members is also enhanced in the secretory phase with respect to the proliferative phase. This indicates the progesterone could be responsible for activating cell-to-cell adhesion[10]. The VEZT promote does not contain a reaction point for the progesterone receptor (PR), but it contains a nuclear factor kappa B (NF-kB) binding site. As a pro-inflammatory transcription factor, NF-kB is involved in the pathogenesis of endometriosis, showing cycle control in the endometrium and reciprocal management with PR[15]. Considering the studied physiological roles of VEZT, its potential for a functional role in endometriosis is a likely option, since VEZT has been demonstrated to be upregulated in ectopic endometrium with respect to eutopic endometrium in patients suffering from endometriosis[11][16][13].
The follicle-stimulating hormone beta polypeptide (FSHB) gene, positioned on chromosome 11 locus 11p14.1, codifies subunit b of the hormone-specific-follicle-stimulating hormone (FSH) with a crucial role in the growth of ovarian follicles and production of estrogens[17][18][19]. Recently, some evidence for an association between endometriosis and SNPs of FSHB was reported in independent targets from the UK Biobank, firmly supporting this result[18]. FSH and luteinizing hormone (LH) are related gonodotropin hormones sharing the same alpha subunit. A connection between these SNPs on chromosome 11 with concentrations of both hormones indicates a common mechanism of regulation, with both being key elements in managing follicle development in the ovary, influencing estradiol release during the proliferative phase of the cycle and contributing to a role for estradiol in endometriosis risk[17]. Data from the ENCODE project show that the SNP rs11031006 modifies the sequence of 11 protein-binding motifs, including that of estrogen receptor alfa, with a possibile effect on hormonal feedback inhibition. Recently, allele G of this SNP has been proven to be significantly associated with higher levels of serum FSH[19].
References
- Fernanda Mafra; Michele Catto; Bianca Bianco; Caio Parente Barbosa; Denise Maria Christofolini; Association of WNT4 polymorphisms with endometriosis in infertile patients. Journal of Assisted Reproduction and Genetics 2015, 32, 1359-1364, 10.1007/s10815-015-0523-1.
- Luca Pagliardini; Davide Gentilini; Paola Viganó; Paola Panina-Bordignon; Mauro Busacca; Massimo Candiani; Anna Maria Di Blasio; An Italian association study and meta-analysis with previous GWAS confirmWNT4,CDKN2BASandFN1as the first identified susceptibility loci for endometriosis. Journal of Medical Genetics 2012, 50, 43-46, 10.1136/jmedgenet-2012-101257.
- Vaino S; Heikkila M; Kispert A; Female Development in Mammals is Regulated by Wnt-4-Signaling. The Endocrinologist 1999, 9, 323, 10.1097/00019616-199907000-00014.
- R Gaetje; Uwe Holtrich; Knut Engels; Stefan Kissler; Achim Rody; Thomas Karn; Manfred Kaufmann; Endometriosis may be generated by mimicking the ontogenetic development of the female genital tract. Fertility and Sterility 2007, 87, 651-656, 10.1016/j.fertnstert.2006.07.1533.
- J. Sundqvist; A. Vodolazkaia; A. Fassbender; C. Kyama; A. Bokor; K. Gemzell-Danielsson; T. M. D'hooghe; Henrik Falconer; H. Xu; Replication of endometriosis-associated single-nucleotide polymorphisms from genome-wide association studies in a Caucasian population. Human Reproduction 2013, 28, 835-839, 10.1093/humrep/des457.
- Zhangying Wu; Ming Yuan; Yan Li; Fangfang Fu; Wenqinq Ma; Haixia Li; Wenwen Wang; Shixuan Wang; Analysis of WNT4 polymorphism in Chinese Han women with endometriosis. Reproductive BioMedicine Online 2015, 30, 415-420, 10.1016/j.rbmo.2014.12.010.
- Nilufer Rahmioglu; Dale R. Nyholt; Andrew P. Morris; Stacey A. Missmer; Grant Montgomery; Krina T. Zondervan; Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Human Reproduction Update 2014, 20, 702-16, 10.1093/humupd/dmu015.
- Dale R. Nyholt; Siew-Kee Low; Carl A. Anderson; Jodie N. Painter; Satoko Uno; Andrew P. Morris; Stuart MacGregor; Scott D. Gordon; Anjali Henders; Nicholas G. Martin; et al.John R. AttiaElizabeth G. HollidayMark McEvoyRodney J. ScottStephen H. KennedySusan A. TreloarStacey A. MissmerSosuke AdachiKenichi TanakaYusuke NakamuraKrina T. ZondervanHitoshi ZembutsuGrant Montgomery Genome-wide association meta-analysis identifies new endometriosis risk loci. Nature Genetics 2012, 44, 1355-1359, 10.1038/ng.2445.
- Satoko Uno; Hitoshi Zembutsu; Akira Hirasawa; Atsushi Takahashi; Michiaki Kubo; Tomoko Akahane; Daisuke Aoki; Naoyuki Kamatani; Koichi Hirata; Yusuke Nakamura; et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nature Genetics 2010, 42, 707-710, 10.1038/ng.612.
- Sarah Holdsworth-Carson; Jenny Nga Ting Fung; Hien T.T. Luong; Yadav Sapkota; Lisa M. Bowdler; Leanne Wallace; Wan Tinn Teh; Joseph E. Powell; Jane E Girling; Martin Healey; et al.Grant MontgomeryP.A.W. Rogers Endometrial vezatin and its association with endometriosis risk. Human Reproduction 2016, 31, 999-1013, 10.1093/humrep/dew047.
- Polonca Küssel-Andermann; Aziz El-Amraoui; Saaid Safieddine; Sylvie Nouaille; Isabelle Perfettini; Marc Lecuit; Pascale Cossart; Uwe Wolfrum; Christine Petit; Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin–catenins complex. The EMBO Journal 2000, 19, 6020-6029, 10.1093/emboj/19.22.6020.
- Orest William Blaschuk; Tracey M. Rowlands; Plasma membrane components of adherens junctions (Review). Molecular Membrane Biology 2002, 19, 75-80, 10.1080/09687680210132467.
- Luca Pagliardini; Davide Gentilini; Ana Maria Sanchez; Massimo Candiani; Paola Viganó; Anna Maria Di Blasio; Replication and meta-analysis of previous genome-wide association studies confirm vezatin as the locus with the strongest evidence for association with endometriosis. Human Reproduction 2015, 30, 987-993, 10.1093/humrep/dev022.
- Juliana Meola; Júlio César Rosa E Silva; Daniel Dentillo; Wilson Araújo Da Silva; Luciana Caricati Veiga-Castelli; Luciano Ângelo De Souza Bernardes; Rui Alberto Ferriani; Claudia C. P. Paz; Silvana Giuliatti; Lucia Martelli; et al. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertility and Sterility 2010, 93, 1750-1773, 10.1016/j.fertnstert.2008.12.058.
- Sun-Wei Guo; Nuclear Factor-κB (NF-κB): An Unsuspected Major Culprit in the Pathogenesis of Endometriosis That Is Still at Large?. Gynecologic and Obstetric Investigation 2006, 63, 71-97, 10.1159/000096047.
- Hien Tt. Luong; Jodie N. Painter; Yadav Sapkota; Dale R. Nyholt; Peter A. Rogers; Grant W. Montgomery; AB028. Identifying the functional role of VEZT gene for endometriosis risk. Annals of Translational Medicine 1970, 3, 1, 10.3978/j.issn.2305-5839.2015.AB028.
- Yadav Sapkota; Valgerdur Steinthorsdottir; Andrew P. Morris; Amelie Fassbender; Nilufer Rahmioglu; Immaculata De Vivo; Julie E. Buring; Futao Zhang; Todd Edwards; Sarah Jones; et al.Dorien ODaniëlle PeterseKathryn M. RexrodePaul M. RidkerAndrew J. SchorkStuart MacGregorNicholas G. MartinChristian M. BeckerSosuke AdachiKosuke YoshiharaTakayuki EnomotoAtsushi TakahashiYoichiro KamataniKoichi MatsudaMichiaki KuboGudmar ThorleifssonReynir T. GeirssonUnnur ThorsteinsdóttirLeanne WallaceThomas M. WergeJian YangDigna R. Velez EdwardsMette NyegaardSiew-Kee LowKrina T. ZondervanStacey A. MissmerThomas D'hoogheGrant MontgomeryDaniel I. ChasmanKári StefánssonJoyce Y. TungDale R. Nyholt Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nature Communications 2017, 8, 15539, 10.1038/ncomms15539.
- Katherine S. Ruth; Robin N. Beaumont; Jessica Tyrrell; Samuel E. Jones; Marcus A. Tuke; Hanieh Yaghootkar; Andrew R. Wood; Rachel M. Freathy; Michael Weedon; Timothy M. Frayling; et al.Anna Murray Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Human Reproduction 2016, 31, 473-81, 10.1093/humrep/dev318.
- Michail Matalliotakis; Maria I. Zervou; Charoula Matalliotaki; Nilufer Rahmioglu; George Koumantakis; Ioannis Kalogiannidis; Ioannis Prapas; Krina T. Zondervan; D.A. Spandidos; Ioannis Matalliotakis; et al.G N Goulielmos The role of gene polymorphisms in endometriosis. Molecular Medicine Reports 2017, 16, 5881-5886, 10.3892/mmr.2017.7398.
