The endoplasmic reticulum (ER) is an important subcellular organelle, primarily recognized as a checkpoint for protein folding. It plays an essential role in ensuring the proper folding and maturation of newly secreted and transmembrane proteins. Different processes are activated when around one-third of newly synthesized proteins enter the ER in the eukaryote cells, such as glycosylation, folding, and/or the assembling of these proteins into protein complexes. However, protein folding in the ER is an error-prone process whereby various stresses easily interfere, leading to the accumulation of unfolded/misfolded proteins and causing ER stress. The unfolded protein response (UPR) is a process that involves sensing ER stress. Many strategies have been developed to reduce ER stress, such as UPR, ER-associated degradation (ERAD), and autophagy.
- plants
- ER
- ER stress
- UPR
- IRE1
- bZIP17
- bZIP28
- bZIP60
1. Introduction
2. Endoplasmic Reticulum (ER)
ER is a large, complex, and highly dynamic cytoplasmic membrane system of eukaryotic cells and is considered to be a central network of interconnected tubules and flattened cisternae that extend across the cytoplasm [7][8][7,8]. The ER network occupies a significant portion of the cytoplasm with its membrane, accounting for ~50% of total cellular membranes [9]. ER plays a crucial role in protein synthesis, peptide chain folding and processing, post-translational modifications, lipid biosynthesis, Ca2+ storage and homeostasis, and the regulation of glucose concentration [10][11][10,11] (Figure 1). This organelle provides an oxidative environment to facilitate the formation of a disulfide bond and is loaded with molecular chaperones [12]. In addition, the ER is involved in regulating the stress responses in animal and plant cells [13].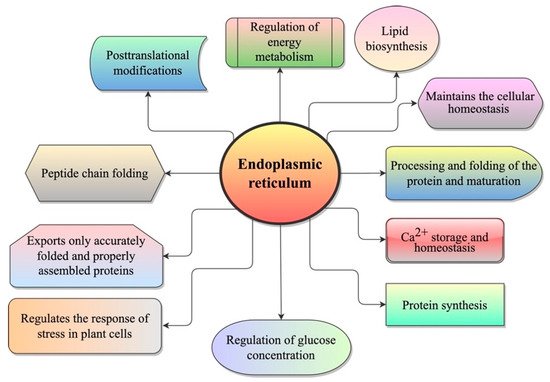
3. ER Stress
4. Chemical Inducers for the Accumulation of the Unfolded Protein
4.1. Tunicamycin (TM) Stress
4.1. Tunicamycin (TM) Stress
4.2. Dithiothreitol (DTT) Stress
4.2. Dithiothreitol (DTT) Stress
5. UPR Signaling in Plant Development
UPR has been found to play a crucial role in both reproductive and vegetative development [14]. Additionally, UPR plays an unexpected role in hormone biology, which may explain the effect of UPR on vegetative growth and development [14]. Normal plant growth and development require UPR. Normal growth and development require the mobilization of basic leucine zipper 17 (bZIP17) into the nucleus [43]. The triple mutant inositol-requiring enzyme 1 (IRE1a IRE1b) bZIP17 grew abnormally under normal growth conditions and was also defective in the stress signaling pathways. The functions of bZIP17 in A. thaliana development were observed in another study using genomic and genetic approaches. In contrast to bZIP28 and bZIP60, the bZIP17 is not a primary UPR activator, but works in conjunction with bZIP28 to regulate development-related genes, particularly stress maintenance and root elongation [44]. In A. thaliana, ER stress induces the expression of NAC103. NAC103 overexpression has pleiotropic effects on plant growth, plays a vital role in inducing the expression of some UPR downstream genes under normal growth conditions [45].6. UPR Signaling in Different Stresses
The UPR can be activated by various stresses in plants that induce the accumulation of unfolded proteins in the ER lumen [46][47][50,51] (Figure 2). UPR has been involved in the immunity and development of plants and provides defense against different stresses [14][42][44][14,42,44], such as heat [48][52], drought [49][53], salinity [50][51][54,55], osmotic pressure, high light intensity and heavy metals. These stresses disturb protein folding [15][52][15,56]. In addition, UPR is activated under protein synthesis overload conditions when the need for protein folding simply does not meet demands [53][54][57,58]. However, cells commit to programmed cell death (PCD) during the failure of UPR in chronic or unresolved ER stress conditions [23][55][56][57][23,59,60,61]. Biotic agents have more complex effects on the UPR. Various biotic agents have been reported to induce the UPR or require the UPR to infect plants. A study observed that when Nicotiana benthamiana was inoculated with the host-pathogen and non-host pathogens, Pseudomonas syringae and Pseudomonas cichorii, respectively, the host pathogen P. syringae did not induce the expression of bZIP60. At the same time, the expression of bZIP60P was induced by the non-host pathogen, P. cichorii. However, the plants became more susceptible to P. syringae when virus-induced gene silencing (VIGS) was used to silence the bZIP60 in N. benthamiana [58][62]. UPR is induced and required by various plant viruses for successful infection.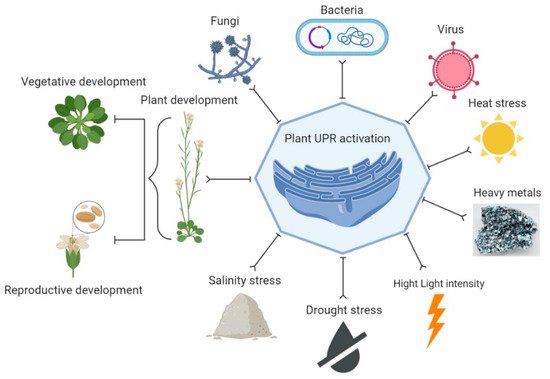
7. Strategies to Reduce ER Stress
7.1. Unfolded Protein Response (UPR)
7.1. Unfolded Protein Response (UPR)
7.2. Mechanism of UPR Signaling Pathway in Plants
7.2. Mechanism of UPR Signaling Pathway in Plants
7.2.1. Regulated IRE-1 Dependent Splicing (RIDS)
7.2.2. ER-Associated Degradation (ERAD)
ERAD is part of the ER protein quality-control system (ERQC), which is considered essential for the conformation fidelity of most of the membrane and secretory proteins in eukaryotes. The ERAD process is associated with the ubiquitin/proteasome system (UPS), which relieves ER stress. The UPS function involves the use of a ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), the ubiquitin ligase (E3), and the 26S proteasome [70][116]. ERAD is accomplished through multistep reactions involving the sequential recruitment of E1, E2, and E3 enzymes. E2 and E3 enzymes are responsible for the specificity of the substrate [71][72][92,117].7.2.3. Autophagy
Autophagy is a self-eating cellular process that has been conserved throughout evolution. Autophagy functions as a degradation process in recycling cellular cytoplasmic contents and removing damaged proteins or organelles under adverse growth conditions [61][101]. There are three main types of autophagy on the basis of their mechanism; chaperone-mediated autophagy [73][119], macroautophagy [74][120], and microautophagy [75][121]. Chaperone-mediated autophagy is highly selective, while macroautophagy and microautophagy may be selective or non-selective [76][77][122,123]. In plants, the following two types of autophagy are known: macroautophagy and microautophagy [78][124]. During macroautophagy, the majority of cytosolic constituents are sequestered into a double-membrane structure, known as an autophagosome. The autophagosome is a specific organelle that mediates macroautophagy. For degradation, autophagy delivers cytoplasmic materials or organelles to the vacuole/lysosome by forming autophagosome [79][80][99,125]. The autophagosome’s outer membrane fuse with the vacuolar membrane upon delivery to the vacuole, delivering the inner membrane structure and its cargo, i.e., the autophagic body into the vacuolar lumen for degradation by vacuolar hydrolases [81][82][126,127].
