Gestational diabetes mellitus (GDM) is the most frequent complication during pregnancy. This complex disease is characterized by glucose intolerance and consequent hyperglycemia that begins or is first diagnosed in pregnancy, and affects almost 7% of pregnant women. Previous reports have shown that GDM is associated with increased pregnancy complications and might cause abnormal fetal development. At present, treatments are not suitable for the prevention and management of these patients. As an alternative therapeutic opportunity and a leading scientific technique, nanotechnology has helped enlighten the health of these affected women. Theranostic nanomaterials with unique properties and small sizes (at least <100 nm in one of their dimensions) have been recently engineered for clinics and pharmaceutics. Reducing materials to the nanoscale has successfully changed their properties and enabled them to uniquely interact with cell biomolecules. Several biosensing methods have been developed to monitor glucose levels in GDM patients.
1. Introduction
Diabetes mellitus (DM) has reached epidemic proportions and is a leading cause of death worldwide (despite the decades of clinical studies and trials of novel therapeutic strategies)
[1]. Because of inaccuracy and insufficiency of data for monitoring DM patients, particularly in developing countries, there is a significant gap in comprehending the burden nationally and globally
[2]. The World Health Organization (WHO) estimated that the prevalence of DM in adults would rise to 300 million cases by 2025
[3]. This number includes patients with GDM and should alert healthcare providers to concentrate on preventive actions before childbirth.
GDM, defined as any level of glucose intolerance with onset or first recognition during pregnancy, affects about 7% of all pregnancies worldwide and poses life-threatening short- and long-term risks for the mother and the baby
[4][5][6][4,5,6]. Inauspiciously, these health consequences emerge at the maternal glucose values
[7]. GDM is characterized by the failure of pancreatic β-cells to respond appropriately to the insulin requirements during gestation, which leads to hyperglycemia
[8]. Obesity, family history of DM, age, and ethnicity are among the main factors that may enhance the risk of GDM
[9]. It has been well established that most women with GDM return to the normoglycemic state soon after childbirth. Until now, the consequences of GDM have extended beyond the pregnancy, with affected women conferring a seven-times increased risk of developing type 2 diabetes mellitus (T2DM) compared with women who maintained normoglycemic during maternity
[10].
For screening GDM, all pregnant women should undergo oral glucose testing with 50-g glucose at 24 to 28 weeks of gestation. If glucose tolerance is impaired, a subsequent glucose tolerance test should be carried out to diagnose GDM
[11]. At present, GDM diagnosis is made by a 75-g or 100-g oral glucose tolerance test
[12]. Still, this test has limitations, and a single test cannot confirm the GDM diagnosis
[11]. Regarding GDM treatment, various efforts have been made to reverse hyperglycemia and decrease the risk of the related adverse pregnancy outcomes
[8]. Furthermore, lifestyle interventions, pharmacological therapies (i.e., insulin therapy and administration of metformin or glibenclamide), and postnatal managements present several therapeutic options associated with the enhanced glycemic control for both the mother and the child
[8][13][14][8,13,14]. With the increasing prevalence of T2DM, specifically in the deprived areas, the precise diagnosis of GDM is now considered an encouraging opportunity for the intervention to alleviate the burden of T2DM
[15]. Accordingly, it seems imperative to develop new theranostic platforms for the accurate diagnosis of this condition.
Recently, the advances in nanomedicine have prompted the designing of favorable therapeutic modalities for various applications
[16][17][18][19][16,17,18,19]. Furthermore, nanomedicine has influenced these efforts by increasing the surface area of the biosensors, enhancing the catalytic properties of the electrodes, and creating nanoscale sensors for a wide range of theranostic purposes
[20]. Nanomaterials, such as NPs
[21][22][23][24][21,22,23,24], block-copolymer micelles
[25], nanocapsules
[26], nanocages
[27], and nanocarriers (i.e., nanoliposomes
[28]), nanocomposites
[29], and nanohydrogels
[30]) with well-controlled properties have emerged for monitoring the blood glucose levels as well as therapy and care of DM and/or GDM patients. These nanomaterials mostly assisted in the direct measurement of glucose in serum or substantially improved the glucose sensor function. Moreover, they acted as the newly developed drug delivery systems (DDSs) to achieve active targeting
[31]. The small-targeted DDSs can ameliorate the severity of DM in patients and promotes the growth and development of pancreatic β-cells via inducing the Wnt signaling pathway, activating the autophagic target points, inhibiting inflammasome, and triggering other molecular pathways
[32]. Various nanosensors, including engineering periplasmic ligand-binding proteins
[33], acetone nanosensors
[34], near-infrared optical nanosensors
[35], copolymer-based fluorescence nanosensors
[36], graphene field-effect transistor nanosensors
[37], silver nanoparticle-modified nanosensors
[38], and other biological nanosensors have been designed as non-invasive diabetes sensing technologies for the sensitive detection of glucose in the affected patients
[39][40][39,40]. For the diagnosis of GDM, intensive development on biomarker sensing is currently being conducted in advanced fields with the help of such nanomaterials.
2. Nanotechnology for Treatment of GDM
As discussed earlier, GDM is a condition of glucose intolerance, in which a person who does not have diabetes will experience hyperglycemia during pregnancy. Therefore, the onset and first diagnosis of this diabetes occur during pregnancy. Risk factors include being overweight, a history of previous GDM, a family history of type 2 diabetes, and polycystic ovary syndrome. A blood test is used to diagnosis this type of diabetes
[41][42][61,62]. GDM can occur due to insulin resistance or decreased insulin production. It also increases the incidence of congenital malformations in the fetus. According to research, mitochondrial damage and oxidative stress are the most influential factors in diabetic fetuses
[21].
2.1. Use of Metallic NPs for Treatment of GDM
Cerium is the second element in the lanthanide series in the periodic table. It is one of the rare elements of the planet, often showing a +3-oxidation state, but is also stable in the +4 state. Cerium has no biological role in humans and is not very toxic
[43][63]. Cerium oxide (CeO
2), in combination with oxygen in an NP. formulation, forms an alloy crystal structure that exhibits profound antioxidant properties
[44][45][64,65]. CeO
2 NPs are potential new drugs for oxidative disorders that overcome the weaknesses of previous treatments and ischemic brain damage
[21][46][21,66]. In a study by Vafaei-Pour et al. in diabetic rats, they used nanoceria as an antioxidant to improve fetal diabetes treatment. Diabetes was induced by a dose of streptozotocin and blood glucose levels were calculated on the 0, 5th, 10th, and 15th day of pregnancy. Diabetes was confirmed when the blood glucose concentration reached more than 200 mg/dL. Oxidative stress, pathological parameters, abortion, and live embryos were assessed. Histological studies showed that diabetes causes abortion. Nanoceria treatment inhibited embryonic oxidative stress as well as pathological changes in diabetic rats. Because diabetes has a teratogenic nature, nanocrystals help treat a diabetic fetus through their antioxidant effects. Therefore, early diagnosis of GDM and administration of antioxidants can reduce these complications
[21]. In another study, Vafaei-pour et al. investigated the protective effect of ceria NPs in preventing mitochondrial damage due to GDM After induction of diabetes by streptozotocin and reaching blood glucose above 200 mg/dL on the 16th day of gestation, the embryo was isolated, and the mitochondria were purified by centrifugation. Markers related to mitochondrial damage and oxidative stress were then analyzed. The results showed that treatment with nanoceria at a dose of 60 mg/kg significantly prevented the development of oxidative stress and mitochondrial toxicity (
p < 0.05)
[47][67]. The defensive effect of CeO
2 NPs in diabetic mice was investigated. CeO
2 NPs enhanced the morphological abnormalities of dorsal root ganglion neurons (DRG). Administration of CeO
2 NPs for 8 weeks significantly reduced the ADP/ATP level in diabetic rats compared to non-diabetic rats (
p < 0.001). This study showed that the effect of diabetes was repressed by CeO
2 NPs
[48][68].
Selenium (Se) is present in plants and is a rare element. Selenium deficiency in the body causes various diseases, including diabetes. This element has antioxidant properties, and Se NPs can inhibit tissue oxidation by inhibiting numerous peroxides, protecting lipids and cellular macromolecules from oxidative damage to membranes, growing glutathione peroxidase levels, then thyroxine reductase
[49][50][69,70]. In a study of T2DM mice, Hanaa et al. found that selenium-containing liposomes maintain β-cell integrity, enhance insulin excretion, lower glucose levels, restore the equilibrium of oxidative, antioxidant production, and reduce pancreatitis; therefore, they have antidiabetic properties
[51][71]. Hassan et al. examined the effect of Se NPs and their therapeutic effects on puppies of mothers with GDM, after administration of 5 mg/kg body weight twice a week for one month. Blood-, pancreas-, and kidney-sacrificed puppies were then biochemically analyzed, and tissues were studied. The results showed that puppies of diabetic mothers treated with synthesized NPs displayed good redox parameters (reduction of glutathione and malondialdehyde in tissue samples). The current findings suggested that the Se NPs could counteract the diabetes-related complications in offspring by reorganizing the cellular redox state. Therefore, the present study shows that Se NPs acted protectively in diabetic mothers containing GDM and did not allow their infants to pass
[52][72].
In 2021, Wang et al. designed an antidiabetic drug delivery device by mimicking pancreatic cells. In this study, hollow mesoporous silica nanoparticles with dual-responsive copolymer coatings were used for subcutaneous delivery of glucose. The dual-response glucose drug delivery system involves a combination of pH and H
2O
2 reacting with a bonded copolymer of hollow mesoporous silica nanoparticles (HMSNs), with a microneedle (MN) patch array. Poly (4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzyl acrylate) -b-poly (2-(dimethylamino) ethyl methacrylate) (PPBEM-b-PDM)—the polymer holds the gate and prevents the drug from secreting from the HMSN cavity at the normoglycemic level. Moreover, due to the chemical change of the H
2O
2-sensitive PPBEM block and acid-responsive PDM block on H
2O
2 and pH stimuli, the drug release rate increases significantly. The combination of antidiabetic and glucose oxidase in HMSNs coated with stimulant polymers results in forming a glucose-mediated MN device after deposition of drug-laden nanoparticles to MN Laboratory and in vivo results showed that the MN device has the property of releasing the drug with glucose adjustment, which has a rapid release of the drug at the level of high blood sugar, but the release of the drug at the normoglycemic level is delayed. Therefore, such a drug delivery system can be very effective in treating diabetes
[53][73].
2.2. Use of Polymeric NPs for GDM Treatment
Chitosan (CS) is the second richest polysaccharide in nature next to cellulose. An amino polysaccharide is a linear product gained through alkaline acetylation of chitin (found in the exoskeleton of certain crustaceans for example shrimp, crabs). Chitosan is biocompatible, degradable, and non-toxic. It can chelate with metal ions. As a result of its cationic and high charge density, CS has various applications in preparing materials, such as flocculants, coagulants, food additives, and weight loss/pharmaceutical formulations
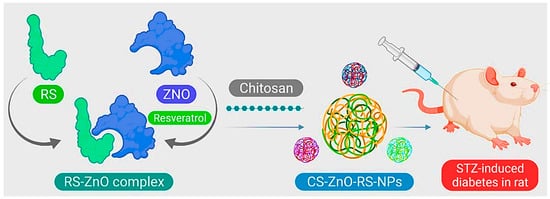
[54][74]. In the study by Du et al., zinc oxide (ZnO)-resveratrol (RS) was encapsulated with CS, and CS-ZnO-RS NPs were synthesized (
Figure 14). Characterization of the NPs by electron microscopy, besides particle analysis, proved that the synthesized CS-ZnO-RS NPs were spherical in shape and had an average size of 38 nm. Moreover, the therapeutic properties of these NPs on GDM were investigated. The results showed that CS-ZnO-RS NPs were able to deliver resveratrol by reducing the side effects and increasing bioavailability. These NPs significantly reduced blood glucose levels, and fat levels in mice with GDM. CS-ZnO-RS NPs at a concentration of 500 μg/mL inhibited α-glucosidase (77.32%) and α-amylase (78.4%). It also reduced the levels of inflammatory agents (IL-6 and MCP-1) in addition to endoplasmic reticulum stress (GRP78, p-IRE1α, p-eIF2α, and p-PERK)
[55][41].
Figure 14. Schematic of CS-ZnO-RS nanoparticle preparation for GDM treatment.
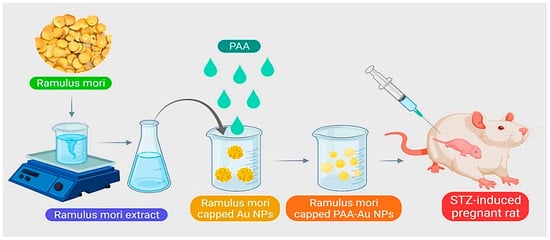
Presently, for the treatment of GDM, it is difficult to deliver drugs accurately and appropriately to the intended treatment site. Uses of gold NPs include use in the treatment of diabetes mellitus, insulin transport, anti-diabetes, and as carriers for delivering various drugs. A research study by Cheng et al. proposed a new method for releasing and producing a diabetic drug. Using the green synthesis method,
Ramulus mori methanolic extract (RME) was loaded on polyacrylic gold NPs (PAA-Au) using chemical polymerization and examined for GDM treatment (
Figure 25). FT-IR results showed the formation of Au-PAA-NPs extract. The results of microscopic observations in diabetic mother rats showed normal variations in liver cell layers. The rat liver received Au NPs and caused significant improvement in liver tissue. Biochemical tests also showed that the use of Au-PAA-NPs improves changes in serum glucose levels in the mother. The present study showed that AuNPs are active in contrast to diabetes. Therefore, it has introduced a new method for treating GDM
[56][75].
Figure 25. Schematic representation for synthesizing polyacrylic gold NPs (PAA-Au NPs) using chemical polymerization to treat GDM.
In another study by Yan et al.,
Murraya koenigii extract (
M. koenigii) and Au-PLGA nanoformulation were synthesized. GDM in rats was induced by streptozotocin (STZ). As a result of treatment with
M. koenigii leaf extract with Au-PLGA nanoformulation, serum levels of lipids and glucose were significantly increased. In pancreas and liver tissue, levels of antioxidant enzymes, due to GDM, were significantly reduced, and levels of cell-strengthening compounds in the pancreas and liver tissue in diabetic rats were the same as in control.
M. koenigii leaf extract, rich in antioxidants, is very effective and can protect cells against chemicals, suppress oxidative blood pressure and insulin and, thus, increase the blood glucose level of GDM in rats
[57][76].