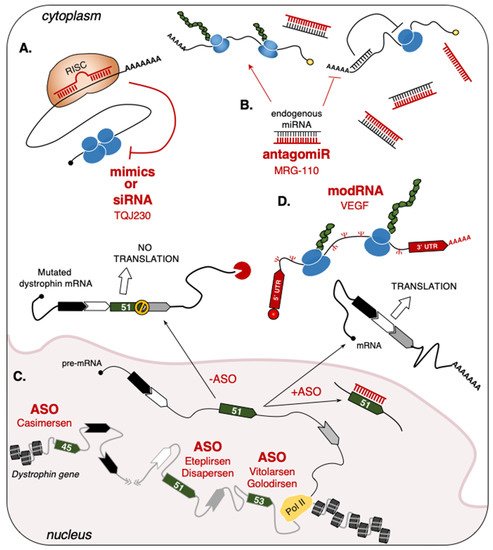
23.1.2. Single-Stranded Antisense Oligonucleotides (ASO)
ASO provided good performances in the treatment of several muscle pathologies
[62][118]. They can be divided into two main categories: The DNA-based ASO, which induces target degradation through the recruitment of RNase H1
[63][119] and the RNA-based ASO, which alters mRNA processing
[64][120] or translation
[65][66][121,122] by a base-pairing block. In 2016 and 2017, respectively, the FDA (Food and Drug Administration) and EMA (European Medicine Agency) agencies approved Spinraza
[67][123], the first RNA-based ASO found to be effective in the treatment of the spinal muscular atrophy (SMA)
[68][124] (
Table 1). In 2016, the FDA also approved Eteplirsen-ASO
[69][125] for use in patients affected by Duchenne Muscular Dystrophy (DMD). This pathology is caused by several types of mutations of the dystrophin gene, which lead to the formation of premature stop-codons in dystrophin mRNA with the consequent loss of protein expression. Over the years, the use of ASO-based drugs able to convert the out-of-frame mutation to in-frame deletions to produce a shorter, but functional, dystrophin protein has been steadily increasing
[70][126]. To date, the exons targeted by this strategy are represented by exon-51 (Eteplirsen, Drisapersen), exon-53 (Vitolarsen, Golodirsen), and exon-45 (Casimersen) (
Figure 12C and
Table 1)
[43][44][45][46][47][93,94,95,96,97]. In particular, Eteplirsen is a 30-nucleotide phosphorodiamidate ASO that induces the skipping of dystrophin exon-51 by impeding the recognition of its splicing sites, thus preventing the formation of a premature stop codon
[69][125]. Even though Eteplirsen was proven to be successful, the treatment can only be applied to ~14% of all DMD patients that present this specific type of mutation
[71][127].
Table 1. RNA-based drugs and biomarkers for cardiac and skeletal pathologies.
| Drug |
RNA Type |
Target |
Disease/Condition |
Company |
Phase |
Reference |
| MRG-110 |
Anti-miR |
miR-92a |
Wound Healing |
miRagen
(Viridian) |
Phase I |
NCT03603431 |
Spinraza
(Nusinersen) |
ASO |
SMN2 |
SMA |
Ionis |
FDA/EMA
approved |
NDA:209531
EMEA/H/C/004312 |
Eteplirsen
(Exondys 51) |
ASO |
Dystrophin |
DMD |
Sarepta
Inotersen |
FDA
approved |
NDA:206488 |
Drisapersen
(Kyndrisa) |
ASO |
Dystrophin |
DMD |
BioMarin |
Phase III |
NCT02636686 |
Vitolarsen
(Viltepso) |
ASO |
Dystrophin |
DMD |
Nippon Shinyaku |
FDA
approved |
NDA:212154 |
Golodirsen
(Vyondis 53) |
ASO |
Dystrophin |
DMD |
Sarepta
Therapeutics |
FDA
approved |
NDA:211970 |
Casimersen
(Amondys 45) |
ASO |
Dystrophin |
DMD |
Sarepta
Therapeutics |
FDA
approved |
NDA:213026 |
| TQJ230 |
siRNA |
Apo(a) |
Cardiovascular Disease,
Elevated Lp(a) |
Novartis |
Phase III |
NCT04023552 |
| AZD8601 |
mRNA |
VEGF |
Ischemic Heart Disease |
Moderna,
Astrazeneca |
Phase II |
NCT03370887 |
| HEARTBiT |
miR |
Biomarker |
Heart Transplant
Rejection |
|
|
NCT03575910 |
| CRUCIAL |
Circulating RNAs |
Biomarker |
Acute Heart Failure |
|
|
NCT03345446 |
While no clinical trial is currently ongoing, promising ASO-based approaches are being applied in mice that model different muscle pathologies, such as centronuclear myopathies
[72][73][74][128,129,130] and myotonic dystrophy type 1 (DM1). DM1 is a multisystemic disorder characterized by myotonia, progressive muscle wasting, cardiac conduction defects, and cognitive impairments
[75][131]. It is caused by the abnormal expansion of CTG repeats in the 3′UTR of
DMPK (dystrophia myotonica protein kinase) transcripts
[76][132] that induces their nuclear retention
[77][133] and sequestration of several RNA-binding proteins, which functional alteration leads to splicing errors
[78][134]. Subcutaneous injection of ASO against DMPK in different DM1 mouse models has yielded positive results in reducing splicing errors, myotonia, and cardiac defects while increasing both skeletal muscle strength
[79][80][135,136] and the number of satellite cells
[81][137], thus facilitating the regeneration process.
23.1.3. Short-Interfering RNA (siRNA)
Other strategies that employ small RNAs are based on the use of small interfering RNAs (siRNAs), which exploit RISC to base-pair and degrade target mRNAs, thus impeding the production of the corresponding protein
[82][141]. Along the years, the efficacy of these molecules has been tested in clinical trials for muscular as well as non-muscular diseases, ranging from polyneuropathy (Patisiran)
[83][142] and chronic hepatitis B viral infection (1JNJ-3989)
[84][143] to different types of cancer, such as pancreatic cancer (siG12D-LODER)
[85][144] and hepatocellular carcinoma (TKM-080301)
[86][145]. In cardiac muscle, these agents are currently being tested in patients with pre-existing cardiovascular diseases. For instance, the administration of TQJ230 siRNAs is shown to inhibit the production of the Apolipoprotein-a (ApoA) and reduce the inflammatory activity of circulating monocytes (
Figure 12A and
Table 1)
[41][87][91,146].
2.2. Long-Sized RNAs
3.2. Long-Sized RNAs
23.2.1. Protein-Coding RNAs
mRNA is the ideal instrument for treatments that require the expression of specific proteins. Over the years, this opportunity has inspired researchers to find new strategies for increasing its stability and minimizing immunogenicity through the modification of specific nucleosides. This culminated with the production of modRNAs, synthetic and chemically modified mRNAs originally applied in phase I and II clinical trials (
https://clinicaltrials.gov accessed on 10 September 2021) to prevent virus infections, such as Coronavirus (NCT04470427), Zika virus (mRNA-1893, NCT04064905; NCT04917861), and Cytomegalovirus (mRNA-1647, NCT04232280), or in the treatment of solid tumors
[88][147]. In cardiac muscle, modRNAs represent a chance for future MI treatments. As for miRNAs, modRNA-based recipes are thought to stimulate cardiomyocytes’ proliferation and increase the blood flow to the wounded area. For example, VEGF-A (Vascular Endothelial Growth Factor-A) is part of a large family of paracrine factors regulating angiogenesis, endothelial cells’ proliferation, and endothelial precursor cells’ differentiation
[89][148]. First tested in cardiac-injured mice
[90][91][149,150], pigs and monkeys
[92][151], the direct delivery of VEGF-A modRNAs through epicardial injection yielded encouraging results in terms of survival, by increasing the density of capillaries surrounding the heart and by reducing apoptotic and scarred areas (
Figure 12D and
Table 1).
23.2.2. Non-Coding RNAs
Cytoplasmic ncRNAs
The functional participation of lncRNAs in muscle regeneration makes them promising targets for clinical applications. In particular, their ability to act as competing endogenous RNAs (ceRNA) attracted the scientific community and currently represents the most exploited way to dose the relative abundance of miRNAs and their targets in vivo (
Figure 23A)
[93][94][95][156,157,158]. Starting from 2011, several lncRNAs were shown to act in the cytoplasm of muscle cells as miRNA sponges
[96][65]. One of the first studies led to the identification of linc-MD1, a lncRNA that governs the timing of skeletal muscle differentiation by sponging miR-133 and miR-135
[97][159]. Upon induction of myoblasts’ differentiation, linc-MD1 starts to be transcribed and inhibits miR-133 and miR-135 activities on their respective targets, MAML1 (Mastermind like transcriptional coactivator 1) and MEF2C (Myocyte enhancer factor 2C). Both proteins are important factors for the transcriptional regulation of pro-differentiating genes
[98][160], thus the ncRNA-mediated regulation of their expression is essential for the correct induction of the latest stages of myogenesis.
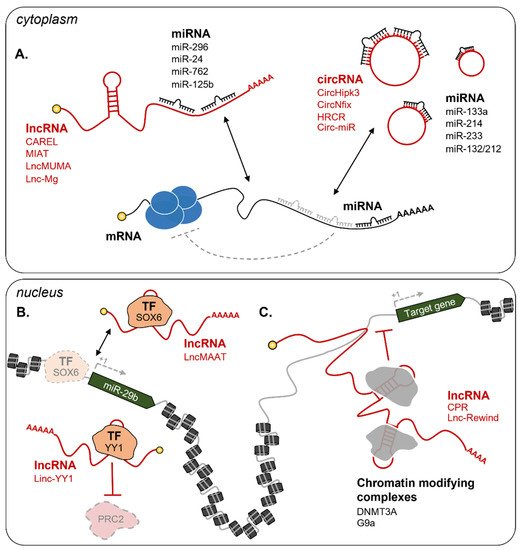
Figure 23. Examples of lncRNA circuitries in muscle regeneration. (
A) In the cytoplasm, lncRNAs and circRNAs can act as competing endogenous RNAs (ceRNA) to interfere with miRNA binding to their targets. Examples include CAREL/mir-296
[99][161], MIAT/miR-24
[100][162], LncMUMA/miR-762
[101][163], Lnc-Mg/miR-125b
[102][164], CircHipk3/miR-133a
[103][165], CircNfix/miR-214
[104][166], HRCR/miR-233
[105][167], and Circ-miR/miR-132/212
[106][168]. In the nucleus, lncRNAs can influence gene expression at the epigenetic level through several mechanisms
[96][65]. Examples in the figure include (
B) lncRNA decoys: LncMAAT impedes SOX6 binding on the promoter of miR-29b to repress its transcription
[107][169], Linc-YY1 binds YY1 and blocks its interaction with the PRC2 complex
[108][170]; (
C) lncRNA guides: CPR
[109][171] and Lnc-Rewind
[110][172] respectively interact with the DNMT3A and G9a repressive complexes and guide them on specific promoters. Dashed grey lines represent the loss of interaction and regulation. TF = Transcription Factor. See text for further details.
Nuclear ncRNAs
The ability to act as sponges is mostly executed by cytoplasmic RNAs. Nuclear and chromatin enriched lncRNAs act as epigenetic rheostats of myogenesis through a variety of mechanisms
[111][96][62,65]. Among the most recent examples, LncMAAT (Muscle-Atrophy-Associated Transcript) is a lncRNA that inhibits miR-29b transcription by impeding the binding of the transcription factor SOX6 to its promoter (
Figure 3B). LncMAAT overexpression has been proposed as a possible strategy to treat muscle atrophy due to the significant attenuation of the pathological phenotypes (i.e. decreased weight of gastrocnemius muscles and grip strength and increased apoptosis) observed in AngII-induced atrophy mice
[107][169]. Another example of a nuclear regulator is CPR (cardiomyocyte proliferation regulator), a lncRNA acting as a guide for the inhibitory DNMT3A (DNA methyltransferase 3 alpha) factor on the promoter of MCM3 (Minichromosome Maintenance Complex Component 3), whose expression is essential for genome replication and cell cycle progression (
Figure 23C)
[109][112][171,185]. In CPR knock-out mice, cardiomyocytes appear smaller than wild-type ones, although they are equipped with higher renewal capability. Indeed, upon MI, these mice show a higher percentage of proliferating cardiomyocytes accompanied by a clear improvement in cardiac functions, as compared to control animals
[109][171]. Contrarily, the lncRNA Linc-YY1 acts as a decoy for YY1 (Yin-Yang 1) by blocking its interaction with the PRC2 complex, leading to the deregulation of several pro-differentiation genes (
Figure 23B)
[113][186]. Depletion of Linc-YY1 by siRNAs in satellite cells caused a significant decrease of MyoG and Pax7 positive cells. This result was also mirrored in vivo in cardiotoxin-induced mice in addition to a reduced number of newly formed myofibers
[108][170]. A further example is Lnc-Rewind (Repressor of wnt induction), a chromatin-associated lncRNA previously identified by transcriptomic analysis
[114][187] and recently shown to act as an epigenetic regulator of satellite cells proliferation and expansion
[110][172]. Mechanistically, Lnc-Rewind directly interacts with the methyltransferase G9a to mediate the repression of its neighboring gene, Wnt7b, the expression of which is important for satellite cells’ differentiation (
Figure 23C).
3. RNA as a Diagnostic Molecule for Muscle Diseases
Together with clinical treatment, the possibility to identify a pathological condition quickly and precociously is extremely important to prevent the worst outcomes. For this reason, studies aimed at the identification of specific RNA biomarkers for different diseases have been steadily growing in the latest years. Both coding and ncRNAs have been found in nearly all peripheral bodily fluids
[115][116][192,193] and could help fill the void of reliable biomarkers.
In muscular diseases, the measurement of circulating biomarkers can lead to extremely rapid, non-invasive, and easy-to-perform diagnostic paths, which overcome the need for surgical biopsies. An increasing number of studies have demonstrated the validity of using circulating miRNAs as biomarkers for muscular disorders, such as DMD and DM1. For instance, the expression of miR-1, miR-206, and miR-133 myo-miRs was found to be high in the serum of DMD patients and strongly correlated to disease severity
[117][118][194,195]. However, as their expression decline with age
[119][196], probably due to the progressive loss of skeletal muscle mass, it is extremely hard to use them as markers in patients. Indeed, it is difficult to discriminate whether their levels are reduced during the pathology due to treatment or age. Another miRNA, miR-483-5p, has been added as a potential biomarker for DMD. Even though it has a lower predictive power in respect to myo-miRs, miR-483-5p expression levels are unchanged with age, thus offering an advantage in monitoring the progress of treated patients
[120][197]. Together with myo-miRs, the pool that includes miR-27b, miR-140-3p, miR-454 and miR-574 can significantly discriminate DM1 patients from healthy controls if analyzed in combination or alone
[121][80]. Their abundance in plasma correlates well with skeletal muscle strength and the levels of creatine kinase, which confirm the potential of miRNAs as biomarkers.
45. Conclusions and Perspectives
RNA shows incredible potential for both diagnosis and treatment of a vast number of diseases, including muscular and cardiovascular pathologies. The use of RNA-based drugs has several advantages, mainly (i) the quick and easy method of design, (ii) the high specificity in target recognition, mostly achieved by base-pairing, (iii) the possibility to target specific cell types or tissues, and (iv) their functional versatility. The usefulness and reliability of small ncRNAs-based drugs (i.e., miRNA, ASO, and siRNA) have already been recognized by the competent FDA and EMA institutions, which have given approval for their use in SMA and DMD. Long RNAs also represent appealing candidates for the development of innovative approaches. Chemical modifications to improve mRNA stability and prevent its immunogenicity have also allowed researchers to pursue their use for the treatment of several conditions. As of now, clinical trials that are being conducted to test the effect of modRNAs expression are still in Phase I or II; however, they already show promising results for future use in human patients. Among the non-coding species, lncRNA-based drugs could be exploited to directly target the nucleus, thus influencing the early stages of gene expression, such as gene transcription, epigenetic regulation, and RNA processing. Despite several studies demonstrating the feasibility of using these molecules for therapeutic purposes in animal models, their application in human patients is still far from being tested. Nevertheless, it is undeniable their potential to revolutionize, in the future, the approaches to therapeutic treatments.