With the rapidly growing human population and changing global climate conditions, it is critical to prevent global crop losses to meet the increasing demand for food and other crop products. The reactive gaseous signaling molecule nitric oxide (NO) is involved in numerous plant developmental processes as well as plant responses to various abiotic stresses through its interactions with various molecules. Together, these interactions lead to the homeostasis of reactive oxygen species (ROS), proline and glutathione biosynthesis, post-translational modifications such as S-nitrosylation, and modulation of gene and protein expression. Exogenous application of various NO donors positively mitigates the negative effects of various abiotic stressors.
- abiotic stress
- nitric oxide (NO)
- drought stress
- heavy metal stress
- soil salinity
- reactive oxygen species (ROS)
- plant stress
1. Introduction
| Plant Species | Drought Imposition | Concentration and Source of NO | Plant Response to NO | Reference |
|---|
| Table | Source and Concentration of Metal | Source and Concentration of Exogenous NO | Plant Species | Impact of NO Treatment | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Citrullus lanatus (watermelon) | 15% PEG 600 | 100 µM SNP | Reduction in MDA content Increased activity of APX Reduced oxidative damage Increased proline content |
[37] | |||||
| A: Copper | 200, 400 µM CuSO4 | 200, 300 µM SNP | Lactuca sativa | Decreased DNA methylation Decreased genomic template instability Increased POX and SOD activity |
[43] | Glycine max | Withholding water | 100 µM SNP | Reduced water loss and improved biomass due to alteration of stomatal characteristics and hydraulic conductivity |
| 5, 25, 50 μM CuSO4 | 10 μM SNP | Arabidopsis thaliana | Increased cell viability[38] | ||||||
| [ | 44 | ] | Origanum majorana | Withholding water | 30 and 60 µM SNP | Improved water use efficiency Increased anthocyanin, soluble phenol, and flavonoid content Enhanced antioxidant capacity |
[39] | ||
| 200 µM CuCl2 | 100 μM SNP | Lolium perenne | Increased activity of SOD, CAT, APX and POX Increased chlorophyll content and photosynthesis Maintenance of Ion homeostasis |
[45] | Brassica juncea | 10% PEG 6000 | 100 µM SNP | Antioxidant accumulation | |
| 0.2 mM Cu | 0.05 mM SNPReduction in MDA content Decreased ROS content |
[40] | |||||||
| Nicotiana tabacum | Increased chlorophyll content, RUBISCO activity and fresh weight | [46] | Triticum aestivum | 15 and 30% PEG | 0.5 mM SNP | Improved antioxidant defence Enhanced glyoxalase system resulting in restoration of leaf relative water content and proline content Enhanced endogenous NO production |
|||
| 450 µM CuSO4 | 200 µM SNP | Hordeum vulgare | Enhanced antioxidant enzyme activity and reduced lipid peroxidation[41] | ||||||
| Activation of AsA-GSH cycle | Withholding water | 50, 100, 150, and 200 µM SNP | 100 µM SNP had a positive impact on chlorophyll content and water status Increased activity of CAT, SOD, and APX Improved activities of GR, GST, GOPX, nitrite and nitrate reductase activity |
[42] |
| [ | |||||
| 47 | |||||
| ] | |||||
| Zea mays | |||||
| B: Cadmium | 150 μM Cd | 150 μM SNP | Hordeum vulgare | Decreased H2O2 and O2− contents Increased AsA, and GSH content Increased expression of HvAOX1a gene |
[48] |
| 200 μM CdSO4 | 200 μM SNP | Catharanthus roseus | Increased melatonin and endogenous NO concentration Increased activities of CAT, SOD, POX Decreased H2O2 and lipid peroxidation in roots |
[49] | |
| 100 μM CdSO4 | 50 μM SNP | Oryza sativa | Decreased Cd uptake by roots Restores RNS/ROS balance |
[50] | |
| 5, 7, or 9 μM CdCl2 | 300 μM SNP | Vigna radiata | Improvement adventitious formation in hypocotyl cuttings Prevents lipid peroxidation Enhanced antioxidant enzyme activity |
[51] | |
| 150 μM | 100 μM SNP | Solanum lycopersicum | Reduced Cd uptake Enhanced AsA-GSH cycle Increased activities of SOD, CAT, GR, MDHAR and APX |
[52] | |
| C: Arsenic | 75 mg/kg (NaAsO2) | 100 μM SNP | Brassica juncea | Increased activities of antioxidant enzymes Increased thiol and proline biosynthesis Decreased As uptake |
[53] |
| 50 μM (Sodium arsenate) |
100 μM SNP | Brassica seedlings | Recovery of photosynthetic pigments Increased CAT and SOD activity resulting in decreased H2O2 and Recovery of AsA and GSH content |
[54] | |
| 150 μM (Sodium meta arsenite) | 100 μM SNP | Oryza sativa | Enhanced nitrogen and thiol content Improved nitrate reductase and GOGAT activity Improved amino acid content |
[55] | |
| 1.5 mg L−1 As | 0.1 mg L−1 SNP | Pistia stratiotes Leaves |
Reduced ROS content Improved photochemical efficiency of PSII Maintained the integrity of cell organelles |
[56] | |
| D: Zinc | 500 µM ZnSO4.7H2O | 100 μM SNP | Carthamus tinctorius | Reduced Zn translocation from root to shoot Enhanced activity of AsA-GSH cycle and glyoxalase system enzymes |
[57] |
| 100, 200 µM ZnO nanoparticles | 100 μM SNP | Triticum aestivum | Decreased Zn accumulation in xylem and phloem saps Improved activity of AsA-GSH cycle |
[58] | |
| 0.05, 0.5 mM Zn (zinc sulfate) in nutrient solution | 0.1 mM SNP | Zea mays | Increased chlorophyll content Decreased leaf and root Zn content Increased nitrogen and iron content |
[59] |
2. Nitric Oxide (NO) Signaling under Abiotic Stresses
2.1. NO and Drought Stress
2.1. NO and Drought Stress
There is credible evidence that NO functions as a vital signaling molecule during both normal growth and development, and drought stress; exogenous application of NO mitigates the negative effects of drought, as seen in soybean, cucumber, and many other plants [38][60][61][30,61,62]. NO has been shown to mediate drought tolerance by activating the ROS scavenging enzyme system [62][63] and increasing osmolyte and proline metabolism [63][64]. NO also modulates water loss through abscisic acid-mediated stomatal response by acting as a secondary messenger in various signaling pathways, such as cyclic guanosine monophosphate (cGMP), mitogen-activated protein kinase (MAPK), and Ca2+ pathways [64][65].
2.1.1. NO and ROS-Mediated Oxidative Stress
During drought stress, plants produce excessive amounts of ROS (oxidative burst) due to a decrease in photosynthesis, leading to an excessive reduction in the electron transport chain and subsequent photooxidative stress [65][72]. NO mitigates the deleterious effects of ROS by limiting lipid peroxidation, increasing the rate of photosynthesis, and promoting antioxidants through various signaling pathways, e.g., the MAP kinase pathway [66][67][77,78]. Drought tolerance of plants is significantly enhanced by the activation of antioxidant enzyme systems such as CAT, SOD, GOPX, APX, DHAR, and GR [68][79]. The activity of CAT and SOD is down-regulated under drought, while application of NO up-regulates antioxidant activity as seen in hull-less barley [69][60]. The metalloenzyme SOD catalyzes the dismutation of superoxide to form H2O2, which is then converted to H2O and O2 by CAT and POX [70][80]. Activation of different isoforms of SOD under drought is considered as a strategy to counteract superoxide anion (O2−) accumulation in different cellular compartments [71][72][81,82]. SOD is quite important in preventing the reaction of O2− with proteins, with unsaturated fatty acids for peroxidation, or with NO to form ONOO−; thus, transgenic plants overexpressing Cu or Zn isoforms of the SOD gene from PuccineliaPuccinelia tenuiflora tenuiflora have increased drought tolerance [73][83]. Fan et al. [74][66] showed that treatment with the NO donor, i.e., sodium nitroprusside (SNP), under drought, up-regulated the activities of SOD, CAT, and POX, resulting in lower ROS accumulation in such plants (Table 1). Increased malondialdehyde (MDA) content and electrolyte loss are important indicators of oxidative damage to cell membranes [75][84], and application of NO can counteract the negative effects of drought by reducing electrolyte loss and decreasing leaf H2O2 and MDA content [69][60]. NO generation has been reported to be up-regulated in Cucumis sativus seedlings upon polyamine (spermine and spermidine) treatment, and its exogenous application in the form of SNP counteracts lipid peroxidation and membrane damage induced by drought stress [8]. Moreover, they also found that exogenous NO application had no effect on endogenous polyamine levels in plants under drought stress but were positively correlated with mitigation of drought induced damage, indicating that polyamines act up-stream of NO in drought stress response.
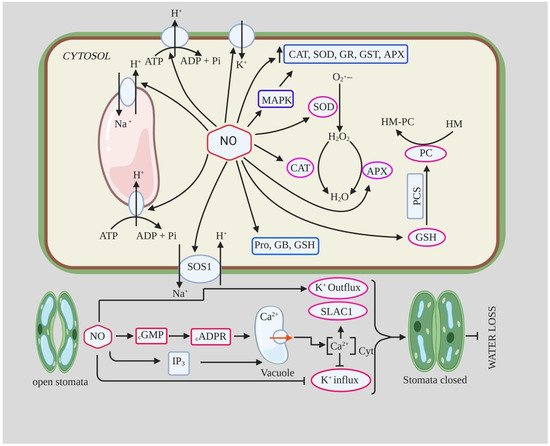
2.1.2. NO and Stomatal Closure during Drought
The exact mechanistic role of NO in ABA-mediated stomatal closure is not yet clear, but it has been proposed that NO is an important component of the ABA signaling pathway for stomatal closure [76][85]. NO acts downstream of the ABA signaling pathway and is an important component of the drought signaling network involved in the control of stomatal transpiration [38][60][30,61]. In a study conducted by Van Meeteren [77][86] on leaves of ViciaVicia faba faba using SNP, NO gas, and ABA, they concluded that NO modifies stomatal opening through several pathways but is probably not critical for rapid ABA-induced stomatal closure. They found that NO/SNP application did not induce stomatal closure in epidermal cells, contradicting previous studies, whereas ABA application did induce stomatal closure [77][86].
2.1.3. NO and Drought-Responsive Genes
Transcription factors are important molecular players that bind to gene promoters to activate or repress transcription. Interestingly, a number of transcription factors are NO-responsive and drought-dependent [78][79][70,100]. Overexpression of SlWRKY8, which belongs to the WRKY transcription factor superfamily, increases drought tolerance in tomato [80][101]. In contrast, SlWRKY81 negatively regulates tomato drought tolerance by repressing NR activity, leading to reduced NO accumulation and eventually to reduced stomatal closure, which in turn increases water loss [81][102]. Silencing of SlWRKY81 resulted in increased NO accumulation in guard cells due to increased NR expression, leading to more efficient stomatal closure and reduced water loss [81][102]. Thus, silencing of SlWRKY81 can be used to increase tolerance in many drought-sensitive plants. Research on NO-mediated gene regulation in plants under drought stress is limited, and further research is needed to fully elucidate its role and paint a more comprehensive mechanistic picture.
2.2. NO and Metal/Metalloid Stress
Naturally occurring metallic elements with relatively higher atomic weight and density than water are called heavy metals (HMs) [82][103]. Contamination of the environment with HMs mostly occurs through anthropogenic activities, such as the use of metals and metal-containing compounds in agriculture and households, mining and smelting, and industrial production [20][83][20,104].
One of the main consequences of HM stress in plants is the excessive ROS formation, due to Fenton and Haber–Weiss reactions and changes in the antioxidant system [84][85][109,110]. Certain metals such as lead and cadmium (Cd) are not directly involved in ROS formation, but they inhibit the antioxidant system and divert electrons from the electron transport chain, indirectly promoting ROS formation [86][87][111,112]. Both endogenous and exogenous NO may play a role in plant perception, signaling, and stress acclimation under HM stress [88][113]. NO is readily diffusible across membranes and is involved in the regulation of numerous physiological processes, including responses to HM stress [89][114].
2.2.1. Cadmium Stress
Cadmium (Cd), a non-essential element and one of the most hazardous pollutants, can be toxic to animals even at non-phytotoxic concentrations [90][116]. It is rapidly taken up by plants due to its high mobility through Fe2+, Ca2+, Zn2+, and Mn2+ transporters, such as the ZIP IRT1 transporter [91][117]. As reviewed by Terrón-Camero et al. [89][114], NO donor application correlates negatively with HM uptake, except for Cd, which showed a positive correlation in about 40% of the studies. Cd accumulation in response to NO could most likely be due to stimulation of IRT1, which has been shown to be NO-dependent and inhibited in the presence of NO synthase inhibitor [92][93][94][118,119,120]. Sharing of IRT1 transporters under Cd stress leads to iron deficiency, which in turn results in NO-mediated up-regulation of FRO2 (Ferric reduction oxidase 2), IRT1 (Iron-regulated transporter 1), and FIT (FER-Like Fe deficiency induced transcription factor), leading to additional Cd accumulation [92][118]. Cd stress has been reported to induce endogenous NO generation, which reduces root growth due to shortening of the root elongation zone, an effect that is reversed by the NOS inhibitor L- NAME (N omega-Nitro-L-arginine methyl ester hydrochloride) [77][86]. However, exogenous NO may prevent the reduction of root growth in response to HM stress [77][86]. NO accumulation under Cd stress leads to the inhibition of root meristem in Arabidopsis due to the reduced AUX level in roots, and this inhibition was alleviated by the application of NO scavengers such as L- NAME and cPTIO (2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) [95][121]. These results suggest that NO has a negative effect on root growth and development under Cd stress. Moreover, the application of NO mitigates the negative effects of Cd stress on plant growth and development [52][96][44,122].
2.2.2. Copper Stress
Cu application has been reported to induce the formation of NO, which is mainly attributed to NOS, one of the most important enzymes in the production of NO [97][130]. However, Hu et al. [47][39] reported that early NO production in HordeumHordeum vulgare vulgare under Cu stress was instead due to the activity of NR (not NOS), as the use of NR inhibitors resulted in decreased NO production in such plants. NO-mediated attenuation of Cu stress could be due to the up-regulation of defense-related genes or antioxidant enzyme activity [98][131]. In addition, NO also maintains the balance of cellular free metal concentrations by controlling their accumulation or excluding HMs in roots [98][131]. Application of SNP to B.B. juncea juncea seeds increased their germination rate and alleviated Cu-induced oxidative stress due to enhancement of the antioxidant system, including SOD, GR, and APX, thereby lowering lipid peroxidation and H2O2 levels [99][132].
2.2.3. Arsenic Stress
Arsenic (As) is a highly toxic metalloid whose toxicity causes various symptoms such as necrosis, decreased photosynthesis, and growth inhibition [87][112]. First, it causes an increase in ROS, leading to increased lipid peroxidation and protein carboxylation, which negatively affect metabolism and disrupt cellular ultrastructure [100][137]. Arsenic also inhibits the activity of enzymes by binding to their sulfhydryl groups (- SH), thereby hindering several important cellular functions [101][138]. Souri et al. [102][139] suggested that As tolerance of IsatisIsatis capadoccica capadoccica (an As hyperaccumulator) may be related to NO as SNP treatment enhances plant growth under As stress, while the application of an NO scavenger and the NOS inhibitor L- NAME reduces plant growth. They concluded that SNP treatment correlated with increased proline, GSH, thiol, and antioxidant concentrations, such as CAT, APX, SOD, and GR, which prevent lipid peroxidation and H2O2 accumulation. GSH, an important thiol compound, is also involved in As tolerance by participating in the biosynthesis of phytochelatin (metal-binding peptide), which binds As III, preventing its toxicity [102][139]. This phytochelatin complex formation is regulated by NO and is considered to be one of the major mechanistic reasons for As hypertolerance in I.I. capadoccica capadoccica [103][140]. The application of SNP to rice plants under As stress can increase primary root length and number of lateral roots compared to As alone, indicating the role of NO in mitigating the effects of As stress on root development [104][141].
2.2.4. Zinc Stress
Accumulation of zinc (Zn) in the environment occurs through both natural causes (volcanic eruptions, fires, and weathering) and anthropogenic activities (electroplating, mining, ore processing, ink and battery industries, and agrochemical application) [105][106][136,147]. Zn plays an important role in various redox reactions and is an essential cofactor of several enzymes, such as SOD, when present in the homeostatic range [107][148]. It is required by plants in trace amounts and is involved in several enzyme-catalyzed reactions; therefore, its toxicity impairs these reactions, which in turn can lead to oxidative stress, senescence, and retarded growth [108][149]. As reported by Kolbert et al. [109][150], Zn stress in Arabidopsis leads to reduced activities of CAT and APX and decreased GSH content, resulting in an overall excess of H2O2.
2.2.5. Other Heavy Metal Stresses (Lead, Chromium, Mercury)
Lead (Pb) is one of the most important environmental pollutants, especially in regions with high anthropogenic activities [110][155], and in toxic concentrations it negatively affects crop biomass as well as yield [111][156]. At high concentrations, it leads to reduced growth, ROS accumulation, irregular phytomorphology, and cell death [112][157]. As reported by Okant and Kaya [113][158], Pb stress leads to increased NO content in maize leaves, and this has also been reported for other plants in previous studies involving different HMs [114][159].
Due to extensive industrial use, chromium (Cr) contamination has become a cause of environmental and scientific concern, with hexavalent Cr(VI) considered the most toxic among its various oxidation states [115][161]. Since it is not an essential element, there is no specific mechanism for its uptake and it competes with sulphur, phosphorus, and iron in carrier binding [116][162]. Once it enters the plants, it causes adverse effects in the plants from molecular level to whole plant level. Huang et al. [117][163] found that NO has the potential to attenuate Cr(VI) toxicity in tall fescue plants, improve the performance of the pigment system II, and improve overall physiological properties in these plants. NO has also been found to be helpful in mitigating Cr(VI)disadvantages in maize seedlings by suppressing lipoxygenase activity and enhancing antioxidant enzyme activities [118][164]. NO has been found to play a crucial role in germination and seedling development under Cr stress. Under Cr(VI)stress, the application of SNP improves seed germination and seedling development of tomato and increases the activity of protease and α-amylase hydrolyzing enzymes [119][165]. Furthermore, they reported an increase in nitrogen, proline, thiol content and antioxidants. These results suggest that exogenous NO application could be useful in Cr phytoremediation.
Mercury (Hg) is a non-essential element and contamination has become a major ecological problem due to the continuous release of Hg into ecological systems due to anthropogenic activities. Hg is introduced into agricultural soils through the use of Hg-containing compounds such as pesticides, fertilizers, lime, and soil amendments, resulting in Hg contamination [120]. Among the various forms of Hg, Hg2.3. NO and Salinity Stress
2.3. NO and Salinity Stress
The role of NO in salt tolerance has been studied in several plant species, and there is ample evidence that application of NO donor protects plants from salt stress by protecting against oxidative stress, maintaining ion homeostasis, regulating osmolyte accumulation, and improving physiological and biochemical parameters [124][125][126][173,174,175]. Treatment of pepper seedlings with 150 mM NaCl resulted in an increase in MDA and H2O2 content by ~100% and 87%, respectively, compared to the control [127][176]. However, they found that foliar application of 150 µM SNP to such seedlings decreased MDA and H2O2 content to 54% and 34%, respectively; it also improved leaf relative water content and antioxidant enzyme activity (SOD, POX, CAT) [127][176]. Ren et al. [126][175] reported that NO (10 µM SNP) pretreatment attenuated the inhibition of seed germination and early seedling growth of BrassicaBrassica chinensis chinensis under salt stress. They found that SNP pretreatment increased antioxidant enzyme activity such as CAT, APX, and SOD and reduced H2O2 and MDA content, which reduced NaCl-induced oxidative damage. They also reported an increase in soluble sugar and proline content and increased K+/Na+ ratio in Radicula and Plumula. The maintenance of high K+/Na+ ratio and reduced Na+ accumulation is important for salt tolerance in plants as they reduce ion toxicity and contribute to the restoration of various metabolic processes [128][177]. The increased K+/Na+ ratio and decreased Na+ accumulation in NO-treated seedlings under salt stress is likely due to the inhibition of vacuolar Na+ compartmentation or Na+ influx through the plasma membrane of radicle [129][178]. Moreover, the increased K+ content and K+/Na+ ratio in NO-treated plants under salt stress could be due to decreased K+ efflux, an increase in competitive absorption sites, increased SOS1 transporter activity, and reduced H2O2 content [128][130][177,179]. In addition, NO was also able to induce the expression of H+-PPase and H+-ATPase, which detoxify the cell through Na+/H+ exchange, as well as the expression of AKT1-type K+ channels, ultimately leading to increased salinity tolerance [131][180]. NO assists sunflower seedlings to adapt to salinity stress (120 Mm NaCl) by regulating polyamine homeostasis by increasing the accumulation of polyamine biosynthetic enzymes, decreasing polyamine catabolism, and regulating their distribution [132][181]. Consistent with this, foliar application as well as pretreatment of NO also alleviates salinity-induced stress in broccoli plants by increasing antioxidant enzyme activity, decreasing MDA and H2O2 content, and improving glycine betaine, phenolics, and chlorophyll-a content [133][182]. NO acts as a cellular preservative that induces the expression of various genes controlling metabolic processes and also alters ROS content [12][134][12,183] (Table 3).
Table 3. Compilation of recent studies on the role of NO in ameliorating plant responses to salinity stress.| Experimental Plant | NaCl Concentration | Concentration and Source of NO | Impact of NO on Plants | Reference |
|---|---|---|---|---|
| Jatropa curcas | 100 mM | 75 μM SNP | Reduced oxidative damage Decreased toxic ion and ROS accumulation Increased accumulation of AsA and GSH Increased activity of CAT, SOD and GR |
[134] |
| Brassica oleracea (Broccoli) |
120 mM | 0.02 mM SNP | Improved CAT, SOD, and POX activity Increased glycine betaine and total phenolic content Reduction in H2O2 and MDA content |
[133] |
| Crocus sativus (Saffron) |
50 and 100 mM NaCl | 10 µM SNP | Improved growth Accumulation of compatible solutes Increased antioxidant enzyme activity and secondary metabolite biosynthesis |
[135] |
| Hylotelephium erythrostictum | 200 mM NaCl | 50 μM SNP | Increased Na+ efflux and decreased K+ efflux Increased Ca2+ influx |
[136] |
| Brassica napus (Rapeseed) |
200 mM NaCl | 10 μM SNP | Redox and ion homeostasis Modulation of antioxidant defence genes SOS2 and NHX1 |
[137] |
| Cicer arietinum L. (chickpea) | 50 and 100 mM NaCl | 50 μM SNAP (S-nitroso-N-acetylpenicillamine) | Increased osmolyte accumulation Upregulation of CAT, SOD and APX genes Decreased electrolyte leakage, MDA and H2O2 content |
[125] |
| Gossypium (Cotton) seedlings | 100 mM NaCl | 0.1 and 1.00 mM SNP | Increased K+ Decreased K+/Na+ ratio Increased antioxidant enzyme activity Decreased MDA content |
[138] |
