Multiple sclerosis (MS) is characterised by widespread damage of the central nervous system that includes alterations in normal-appearing white matter (NAWM) and demyelinating white matter (WM) lesions. Neurite orientation dispersion and density imaging (NODDI) has been proposed to provide a precise characterisation of WM microstructures. NODDI maps can be calculated for the Neurite Density Index (NDI) and Orientation Dispersion Index (ODI), which estimate orientation dispersion and neurite density. Although NODDI has not been widely applied in MS, this technique is promising in investigating the complexity of MS pathology, as it is more specific than diffusion tensor imaging (DTI) in capturing microstructural alterations.
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease that affects almost 2.5 million individuals worldwide, often in young adulthood
[1] MS is characterised by widespread damage of the central nervous system that includes alterations in normal-appearing white matter (NAWM) and demyelinating white matter (WM) lesions
[2].
Magnetic resonance imaging (MRI) plays an essential role in diagnosing and monitoring the disease course and the treatment effectiveness in MS. However, conventional MRI techniques have limited sensitivity to quantify microstructural alterations accompanying neuroaxonal degeneration in brain WM and MS lesions
[3][4][5][3,4,5]. Quantitative MRI biomarkers of the brain provide a more sensitive detection of axonal degeneration and may become a more reliable outcome measure
[6][7][6,7]. Diffusion tensor imaging (DTI) is a quantitative technique that has been widely used to characterise the microstructural abnormalities within both NAWM and WM lesions in MS
[8]. Many studies have reported reduced fractional anisotropy (FA) and increased mean diffusivity (MD) in NAWM in MS patients compared with healthy participants
[9][10][11][12][9,10,11,12] and one meta-analysis has confirmed a significant reduction of FA that suggested a widespread WM damage in MS
[13]. Although these abnormalities may occur early in MS
[14], DTI changes in the NAWM in patients with MS are linked with significant disability
[15]. MRI-histopathological studies have confirmed a high correlation between the DTI changes and axonal count in WM lesions and NAWM, indicating that these abnormalities may reflect pathological alterations related to disability
[16][17][18][16,17,18]. Despite the DTI sensitivity in detecting microstructural changes in WM, the lack of specificity is one of the caveats of DTI. In particular, DTI indices are affected by the orientation dispersion of fibres
[19], which may lead to misinterpretation
[20]. Furthermore, the interpretation of DTI parameters becomes more complex when two or more different tissues with diffusion properties are present in a single voxel
[20].
A biophysical diffusion model known as neurite orientation dispersion and density imaging (NODDI) has been proposed to overcome some DTI limitations, providing a more precise characterization of WM microstructures
[21]. NODDI describes brain tissue as a simplified combination of three compartments. A Watson distribution assumption of sticks models the first compartment, known as the intracellular compartment (axons and dendrites), and the signal of each stick is assumed to be a degenerated diffusion tensor with a perpendicular diffusivity equal to zero. The extracellular compartment is a Gaussian anisotropic diffusion, as seen in DTI, and the free water compartment is an isotropic Gaussian diffusion such as Cerebral Spinal Fluid (CSF). After fitting the NODDI model, maps can be calculated for the Neurite Density Index (NDI), the Orientation Dispersion Index (ODI), and isotropic signal fraction (isoVF). These maps explicitly estimate the orientation dispersion and neurite density, all of which contribute to conventional DTI parameters such as FA
[20]. Although NODDI has not been widely applied in MS, this technique is promising in investigating the complexity of MS pathology, as it is more specific than DTI in detecting microstructural alterations
[20][22][23][24][25][20,22,23,24,25]. Recent studies found that DTI and myelin-sensitive imaging are less sensitive than NODDI to neurite density and changes in NAWM and WM lesions in patients with MS
[23][24][26][23,24,26].
2. Neurite Orientation Dispersion and Density Imaging in Multiple Sclerosis Imaging
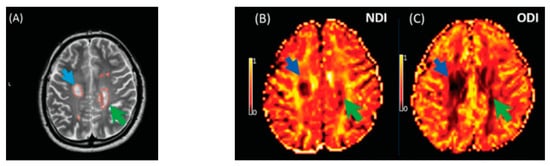
The NDI was reduced in MS lesions and NAWM compared to healthy controls (Figure 18). This significant reduction of the NDI corresponds to reduced intracellular signal fraction and may reflect underlying damage or loss of neurites. Previous work has shown that this reduction is accompanied by higher MD and lower FA [24], and the higher ODI suggests demyelination, axonal loss, and less coherence in the fibre orientation (bending or fanning axons) [23]. This meta-analysis demonstrates a significant reduction of intracellular signal fraction in the NAWM of MS subjects compared with the WM of controls, suggesting early axonal pathology outside demyelination in WM lesions [27][28][29][30][30,31,32,33]. This consistent reduction among all studies proves that the NDI (through its sensitivity to the intra-axonal compartment) may quantify and differentiate microstructural changes in WM MS lesions, in the NAWM of MS, and in healthy WM. This suggests a loss of axonal density in WM lesions and NAWM in MS compared with healthy controls, which is consistent with previous DTI studies [10][31][10,34] reporting altered water diffusion in NAWM and WM MS lesions compared with controlled subjects. Interestingly, the reduction of the NDI in WM lesions and NAWM is in line with previous pathological studies showing axonal loss within WM lesions and a lesser degree in NAWM [17][30][31][32][17,33,34,35]. This consistent reduction of the NDI reported may further assist researchers in relying on NODDI metrics in biophysically understanding the axonal loss/damage in WM lesions and NAWM.
Figure 18. Illustrates NODDI metrics in a single slice of one MS subject. The MS lesion in the major white matter tracts (blue arrow) and periventricular lesion (green arrow) are marked in a structural MRI image (
A), NDI map (
B), and ODI map (
C). Both the NDI and ODI are decreased in the MS lesion
[20].
Few studies reported a reduced ODI within the WM lesions than in healthy WM, suggesting that neurites have less orientation variability or loss of neuronal fibres, which can be more severe in lesions
[20][24][25][20,24,25]. In contrast, the higher ODI within the WM lesions and NAWM, more than in healthy WM, might suggest a loss of fibre coherence and relatively maintained neuronal fibre density
[20][24][25][26][20,24,25,26].
However, our results contradict these reported results, showing no differences in the orientation dispersion of neurites in patients with MS. In addition, the ODI may vary across regions of the brain and can be very diverse across MS subjects, depending on the stage of MS. Therefore, the ODI may be extracted from heterogeneous areas of interest. Most importantly, the variability of b-values/scanning protocols may lead to heterogeneity and influence the ODI findings of this meta-analysis. For this reason, prospective researchers in the field may consider that NODDI metrics require careful interpretation and a study/scanning protocol design.
3. Conclusions
The NDI is significantly reduced in MS lesions and NAWM than WM from healthy participants, which corresponds to the reduced intracellular signal fraction and may reflect underlying damage or loss of neurites. We were unable to demonstrate differences in the ODI in MS lesions and NAWM compared to WM from healthy participants but have identified that heterogeneity in studies may limit the meta-analysis. Further analysis of the NODDI approach in MS for the characterisation of disease-related ultrastructural changes is justified.