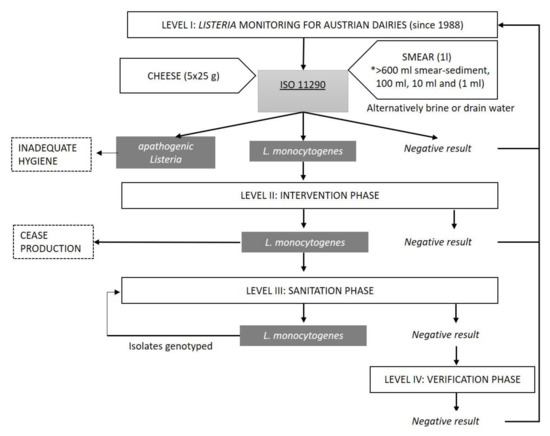
To minimize the risk of process contamination during cheese ripening via the cheese smear, this liquid-based sampling strategy was established, which is also applicable to brine or drain water samples. Since the majority of soft, semi-hard and hard cheeses in Austria are surface-ripened, smear liquids are, in most cases, collected after the smearing process. Compared to product-contact surface-sampling using friction-swabs, these liquids constitute a matrix that provides a much broader representation of the contamination status by including both cheese components and contact with surfaces inside of the production equipment, e.g., smear robots. Sampling of a non-homogenous solid product creates real challenges in terms of consistency and representativeness. Listeria contamination is more likely on the surface rind than inside the cheese matrix. Moreover, sampling of a batch of individual cheeses has potential for statistical biases unless true randomisation is rigorously adhered to. Sampling biases are major concerns and the degree of harmonization among procedures is usually low (sampling frequency and sampling sites are usually less well standardized). The implementation of preventive food safety concepts by tailored food sector-specific sampling procedures provokes a deepened insight of the FBOs into the operation-specific status of contamination and facilitates a comparison of scenarios.
1. Overview
Most Austrian dairies and cheese manufacturers participated in a Listeria monitoring program, which was established after the first reports of dairy product-associated listeriosis outbreaks more than thirty years ago. Within the Listeria monitoring program, up to 800 mL of product-associated liquids such as cheese smear or brine are processed in a semi-quantitative approach to increase epidemiological sensitivity. A sampling strategy within cheese production, which detects environmental contamination before it results in problematic food contamination, has benefits for food safety management. The liquid-based sampling strategy was implemented by both industrial cheese makers and small-scale dairies located in the mountainous region of Western Austria. This report considers more than 12,000 Listeria spp. examinations of liquid-based samples in the 2009 to 2018 timeframe. Overall, the occurrence of L. monocytogenes in smear liquid samples was 1.29% and 1.55% (n = 5043 and n = 7194 tested samples) for small and industrial cheese enterprises, respectively. The liquid-based sampling strategy for Listeria monitoring at the plant level appears to be superior to solid surface monitoring. Cheese smear liquids seem to have good utility as an index of the contamination of cheese up to that point in production. A modelling or validation process should be performed for the new semi-quantitative approach to estimate the true impact of the method in terms of reducing Listeria contamination at the cheese plant level.
2. Cheese
Cheeses made from goat or sheep milk are particularly likely to be
L. monocytogenes positive (3.6–12.8%)
[4]. This is also evident from a search of the portal for Food and Feed Safety Alerts (RASSF), where 39/90
L. monocytogenes notifications relate to cheeses made from goat or sheep milk (
https://webgate.ec.europa.eu/rasff-window/screen/search; accessed on: 19 June 2021). Significant genetic diversity was identified among
L. monocytogenes strains through the use of molecular epidemiology methods
[5][6][7][8][9][10][5,6,7,8,9,10]. Other research groups noticed an increased occurrence of hypervirulent
L. monocytogenes strains of genetic lineage I (serovar 1/2b, 4b, sequence type (ST)1, ST4, ST6) in the dairy niche
[11][12][11,12]. In addition,
L. monocytogenes genetic lineage II strains (e.g., ST7, ST14, ST204; ST451), including hypovirulent types (ST121, ST9) were reported to persist in the dairy processing environment, potentially due to the intra- and inter-species exchange of mobile genetic elements
[6][13][14][15][16][17][18][6,13,14,15,16,17,18].
An important role in environmental adaptation is played by highly conserved plasmids circulating worldwide in a distinctive
L. monocytogenes gene pool
[9][19][20][21][9,19,20,21]. These more complex epidemiological considerations have a direct impact on surveillance used to verify the effectiveness of
L. monocytogenes controls within food safety management systems.
Although milk is usually subjected to a heating process prior to processing, cheese can become contaminated during several process steps such as pressing, curing, ripening, and during cutting and packaging
[22][23][22,23].
In food processing environments (FPEs), contamination is often related to
L. monocytogenes’ colonization of surfaces, including in the dairy sector
[24].
Own-check systems are applied with a focus on testing end products and samples from the production environment according to EC regulation 2073/2005
[25]. In food processing environments (FPEs), contamination is often related to
L. monocytogenes’ colonization of surfaces, including in the dairy sector
[25].
In particular, newly built manufacturing plants or plants undergoing reconstruction measures are at high risk of being colonized with
L. monocytogenes [26][27][26,27].
In cases where L. monocytogenes is detected on the end product at unacceptable levels, withdrawals from the market or recalls are implemented to protect the safety of the consumer.
To minimize the risk of process contamination during cheese ripening via the cheese smear, this liquid-based sampling strategy was established, which is also applicable to brine or drain water samples
[28] (
Figure 1). Since the majority of soft, semi-hard and hard cheeses in Austria are surface-ripened, smear liquids are, in most cases, collected after the smearing process. Compared to product-contact surface-sampling using friction-swabs, these liquids constitute a matrix that provides a much broader representation of the contamination status by including both cheese components and contact with surfaces inside of the production equipment, e.g., smear robots
[29]. Sampling of a non-homogenous solid product creates real challenges in terms of consistency and representativeness.
Listeria contamination is more likely on the surface rind than inside the cheese matrix. Moreover, sampling of a batch of individual cheeses has potential for statistical biases unless true randomisation is rigorously adhered to
[3][30][3,30]. Sampling biases are major concerns and the degree of harmonization among procedures is usually low (sampling frequency and sampling sites are usually less well standardized)
[31]. The implementation of preventive food safety concepts by tailored food sector-specific sampling procedures provokes a deepened insight of the FBOs into the operation-specific status of contamination and facilitates a comparison of scenarios.
Figure 1. Flow chart displaying the structure of the Austrian Listeria monitoring and intervention program. Abbreviations: *, semi-quantitative liquid-based sample quantities.
The monitoring of cheeses produced without smearing focuses on sampling liquids including brine, wash water (water used to clean production devices such as trolleys or trays) or drain water. Sampling events depend on ripening time and batch size and should be performed twice per month. For small-scale dairies, the sampling frequency should ensure that every cheese is included at least once during ripening. After detection of
L. monocytogenes and
Listeria spp. by ISO enrichment methods, PCR-based species differentiation should be performed on typical
Listeria colonies isolated on selective agar
[32][33][32,33]. Persistence of
L. innocua was shown to occur more frequently than persistence of
L. monocytogenes and is, therefore, seen as an indicator of inadequate hygiene
[34][35][34,35].
If L. monocytogenes is detected, rigorous sanitation of the facility is essential. Additionally, the sample number is increased and testing entails end products and further environmental samples (e.g., tanks, racks, conveyor belts and ventilation). This step includes a microbiological investigation post sanitation to verify the efficiency of the measures taken. If desired, a facility inspection audits the internal traffic management and checks other elements of the prerequisite programs (PrPs) that are in place, such as the maintenance of buildings and rooms. The hygienic status of production is, therefore, checked stepwise at all production areas. At the heart of the monitoring and surveillance approach is the range of sample volume that is tested: 600 to 800 mL (two labs involved, method slightly deviates), 100 mL, 10 mL, and 1 mL of liquid (Figure 1). This semi-quantitative way of testing both low and high sample volumes substantially increases the epidemiological sensitivity of the method due to a higher quantity of sample matrix.
Indeed, directly after initial contamination of either the environment or the food, L. monocytogenes might be scarcely detectable in food business operations (FBOs) and testing of high volumes increases the likelihood of finding low contamination levels.
3. Conclusions
The increasing trend of listeriosis incidence in Austria, from a mean value of 0.17 per 100,000 inhabitants from 2000 to 2005 to a mean value of 0.4 from 2009 to 2018, emphasizes the requirements for effective strategies that meet the control needs of the national public health system and food manufacturers. The liquid-based sampling strategy within a Listeria monitoring program at the plant level appears to be superior to solid surface monitoring. Cheese smear liquids seem to have good utility as an index of the contamination of cheese up to that point in production. Multiple volumes of liquid phase, as implemented with our semi-quantitative approach, seem to improve the likelihood of detection, which is consistent with improved epidemiological sensitivity. Monitoring results show a downward trend in Listeria prevalence within this matrix, at least for industrial cheese production, which is thereby consistent with improved hygiene in cheese processing environments and cheese products. Modeling or performance testing of this new semi-quantitative approach against the ISO method would be important to more concretely assess the potential for Listeria minimization in cheese production.