Recently, the bioconversion of biomass into biofuels and biocommodities has received significant attention. Although green technologies for biofuel and biocommodity production are advancing, the productivity and yield from these techniques are low. Over the past years, various recovery and purification techniques have been developed and successfully employed to improve these technologies. However, these technologies still require improvement regarding the energy-consumption-related costs, low yield and product purity. In the context of sustainable green production, this review presents a broad review of membrane purification technologies/methods for succinic acid, a biocommodity obtained from lignocellulosic biomass.
- lignocellulosic biomass
- membrane
- organic acids
- purification
- recovery
- succinic acid
- techniques
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
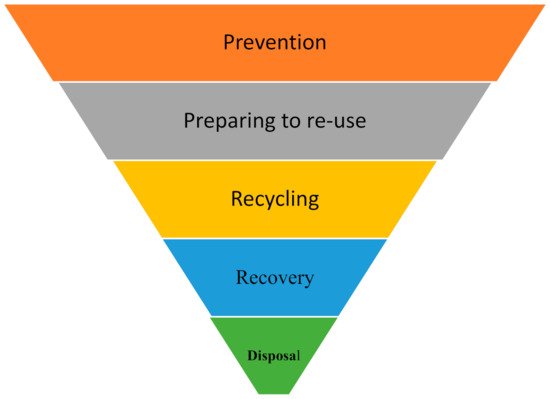
1. Global Market for Sustainable Green Chemistry
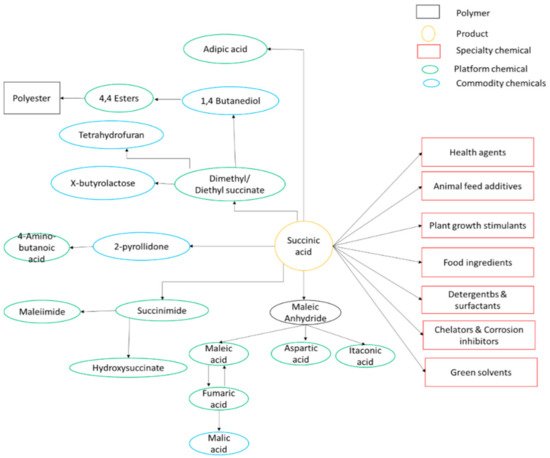
2. Succinic Acid and Its Uses
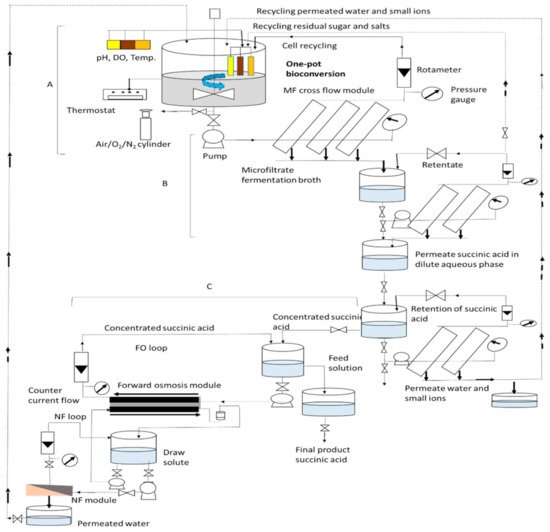
3. Production of SA via “One-Pot” Bioconversion of Biomass with Integrated-Membrane-Based Separation
2. Chen, H.; Wang, L. Chapter 1-Introduction. In Technologies for Biochemical Conversion of Biomass; Academic Press: Cambridge, MA,
USA, 2017; pp. 1–10. [CrossRef]
3. Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11.
[CrossRef]
4. Xie, H.; Gathergood, N. The Role of Green Chemistry in Biomass Processing and Conversion; John Wiley & Sons: Toronto, ON, Canada,
2012; pp. 1–496. ISBN 978-0-470-64410-2. Available online: https://www.wiley.com/en-us/9780470644102 (accessed on 18 May
2021).
5. Bernick, L. The Right Chemistry. The $100 Billion Business Case for Safer Chemistry. Available online: https://www.greenbiz.
com/article/100-billion-business-case-safer-chemistry (accessed on 31 October 2020).
6. Deselnicu, D.C.; Militaru, G.; Deselnicu, V.; Zainescu, G.; Albu, L. Towards a circular economy—A zero waste programme
for Europe. In Proceedings of the CAMS 2018—7th International Conference on Advanced Materials and Systems, Timisoara,
Romania, 28–31 March 2018; pp. 563–568. [CrossRef]
7. Molino, A.; Casella, P.; Marino, T.; Iovine, A.; Dimatteo, S.; Balducchi, R.; Musmarra, D. Succinic Acid Production as Main Player
of the Green Chemistry Industry by using Actinobacillussuccinogens. Chem. Eng. Trans. 2020, 79, 289–294. [CrossRef]
8. Cespi, D.; Esposito, I.; Cucciniello, R.; Anastas, P.T. Beyond the beaker: Benign by design society. Curr. Res. Green Sustain. Chem.
2020, 3, 100028. [CrossRef]
9. Morales, M.; Ataman, M.; Badr, S.; Linster, S.; Kourlimpinis, I.; Papadokonstantakis, S.; Hatzimanikatis, V.; Hungerbühler, K.
Sustainability assessment of succinic acid production technologies from biomass using metabolic engineering. Energy Environ.
Sci. 2016, 9, 2669–2926. [CrossRef]
10. Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and applications of succinic acid. In Current Developments in Biotechnology
and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630.
11. Erickson, B.; NelsonWinters, P. Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol. J.
2012, 7, 176–185. [CrossRef]
12. Law, J.Y.; Mohammad, A.W.; Tee, Z.K.; Zaman, N.K.; Md Jahim, J.; Santanaraj, J.; Sajab, M.S. Recovery of succinic acid from
fermentation broth by forward osmosis-assisted crystallization process. J. Membr. Sci. 2019, 583, 139–151. [CrossRef]
13. Transparency Market Research. The Succinic Acid Market. pp. 1–95. Available online: https://www.transparencymarketresearch.
com/succinic-acid.html (accessed on 13 April 2021).
14. Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol.
2016, 42, 54–66. [CrossRef] [PubMed]
15. Sekoai, P.T.; Awosusi, A.A.; Yoro, K.O.; Singo, M.; Oloye, O.; Ayeni, A.O.; Bodunrin, M.; Daramola, M.O. Microbial cell
immobilization in biohydrogen production: A short overview. Crit. Rev. Biotechnol. 2018, 38, 157–171. [CrossRef] [PubMed]
16. Apergis, N.; Payne, J.E. Renewable energy, output, CO2 emissions, and fossil fuel prices in Central America: Evidence from a
nonlinear panel smooth transition vector error correction model. Energy Econ. 2014, 42, 226–232. [CrossRef]
17. Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.E.; Al-Shannag, M.Wastes and biomass materials as
sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314. [CrossRef]
18. Kumar, R.; Basak, B.; Jeon, B.-H. Sustainable production and purification of succinic acid: A review of membrane-integrated
green approach. J. Clean. Prod. 2020, 277, 123954–123973. [CrossRef]
19. Kurzrock, T.;Weuster-Botz, D. Recovery of succinic acid from fermentation broth. Biotechnol. Lett. 2010, 32, 331–339. [CrossRef]
20. Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse
gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod. Bioref. 2014, 8, 16–29. [CrossRef]
21. Zheng, P.; Fang, L.; Xu, Y.; Dong, J.J.; Ni, Y.; Sun, Z.H. Succinic acid production from corn stover by simultaneous saccharification
and fermentation using Actinobacillus succinogenes. Bioresour. Technol. 2010, 101, 7889–7894. [CrossRef]
22. Willke, T.; Vorlop, K.D. Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl.
Microbiol. Biotechnol. 2004, 66, 131–142. [CrossRef] [PubMed]
23. Beauprez, J.J.; De Mey, M.; Soetaert,W.K. Microbial succinic acid production: Natural versus metabolic engineered producers.
Process Biochem. 2010, 45, 1103–1114. [CrossRef]
24. Li, Q.; Wang, D.; Wu, Y.; Li, W.; Zhang, Y.; Xing, J.; Su, Z. One step recovery of succinic acid from fermentation broths by
crystallization. Sep. Purif. Technol. 2010, 72, 294–300. [CrossRef]
25. Cimini, D.; Argenzio, O.; Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of succinic
acid from Basfia succiniciproducens up to the pilot scale from Arundo donax hydrolysate. Bioresour. Technol. 2016, 222, 355–360.
[CrossRef] [PubMed]
26. Kurzrock, T.;Weuster-Botz, D. New reactive extraction systems for separation of bio-succinic acid. Bioprocess Biosyst. Eng. 2011,
34, 779–787. [CrossRef]
Sustainability 2021, 13, 6794 26 of 30
27. Alexandri, M.; Vlysidis, A.; Papapostolou, H.; Tverezovskaya, O.; Tverezovskiy, V.; Kookos, I.K.; Koutinas, A. Downstream
separation and purification of succinic acid from fermentation broths using spent sulphite liquor as feedstock. Separ. Purif.
Technol. 2019, 209, 666–675. [CrossRef]
28. Pal, P.; Kumar, R.; Chakravarthi, D.V.; Chakrabortty, S. Modelling and simulation of continuous production of L (+) glutamic acid
in a membrane-integrated bioreactor. Biochem. Eng. J. 2016, 106, 68–86. [CrossRef]
29. Kumar, R.; Pal, P. Fermentative production of poly (g-glutamic acid) from renewable carbon source and downstream purification
through a continuous membrane-integrated hybrid process. Bioresour. Technol. 2015, 177, 141–148. [CrossRef]
30. Sosa, P.A.; Roca, C.; Velizarov, S. Membrane assisted recovery and purification of bio-based succinic acid for improved process
sustainability. J. Membr. Sci. 2016, 501236–501247. [CrossRef]
31. Londono, A.O. Separation of Succinic Acid from Fermentation Broths and Esterification by a Reactive Distillation Method. Ph.D.
Thesis, Michigan State University, East Lansing, MI, USA, 2010. Available online: https://d.lib.msu.edu/etd/888. (accessed on
13 July 2020).
32. Awosusi, A.A. Bioconversion of Waste Lignocellulosic Biomass (South African corn cob) to Succinic Acid in Molten Hydrate
Solvent System. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2018. Available online: https:
//hdl.handle.net/10539/25888 (accessed on 24 October 2020).
33. Cornils, B.; Lappe, P. Dicarboxylic Acids, Aliphatic Ullmann’s Enzyklopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2002.
[CrossRef]
34. Li, Q.Z.; Jiang, X.L.; Feng, X.J.; Wang, J.M.; Sun, C.; Zhang, H.B. Recovery processes of organic acids from fermentation broths in
the biomass-based industry. J. Microbiol. Biotechnol. 2016, 26, 1–8. [CrossRef]
35. Agarwal, L.; Isar, J.; Saxena, R. Rapid screening procedures for identification of succinic acid producers. J. Biochem. Biophys. 2005,
63, 24–32. [CrossRef] [PubMed]
36. Cheng, K.K.; Zhao, X.B.; Zeng, J.; Wu, R.C.; Xu, Y.Z.; Liu, D.H.; Zhang, J.A. Downstream processing of biotechnological produced
succinic acid. Appl. Microbiol. Biotechnol. 2012, 95, 841–850. [CrossRef] [PubMed]
37. Song, Y.; Xu, J.; Xu, Y.; Gao, X.; Gao, C. Performance of UF-NF integrated membrane process for seawater softening. Desalination
2011, 276, 109–116. [CrossRef]
38. Huh, Y.S.; Jun, Y.S.; Hong, Y.K.; Song, H.; Lee, S.Y.; Hong,W.H. Effective purification of succinic acid from fermentation broth
produced by Mannheimiasucciniciproducens. Process Biochem. 2006, 41, 1461–1465. [CrossRef]
39. Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem.
Rev. 2006, 106, 4044–4098. [CrossRef] [PubMed]
40. Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels.
Chem. Soc. Rev. 2011, 40, 5588–5617. [CrossRef]
41. Isikgor, F.H.; Becer, R. Lignocellulosic Biomass: A Sustainable Platform for Production of Bio-Based Chemicals and Polymers.
Polym. Chem. 2015, 6. [CrossRef]
42. Saha, B.C. Enzymes as Biocatalysts for Conversion of Lignocellulosic Biomass to Fermentable Sugars. In Handbook of Industrial
Biocatalysis; Hou, C.T., Ed.; CRC Press: Abingdon, UK, 2005.
43. Ladisch, M.; Ximenes, E.; Kim, Y.; Mosier, N.S. Biomass Chemistry and pretreatment for biological processes. In Catalysis for the
Conversion and Its Derivatives; Behrens, M., Datye, A., Eds.; Max Planck Research Library for the History and Development of
Knowledge: Berlin, Germany, 2013; pp. 131–158. ISBN 978-3-8442-4282-9.
44. Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries:
A review. Bioresour. Technol. 2013, 134, 362–373. [CrossRef] [PubMed]
45. Holm, J.; Lassi, U. Ionic Liquids: Applications and Perspectives; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-248-7.
[CrossRef]
46. Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic
biomass. Green Chem. 2013, 15, 584–595. [CrossRef]
47. Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag.
2010, 51, 1412–1421. [CrossRef]
48. Huber, G.W. Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries;
National Science Foundation: Washington, DC, USA, 2008; p. 180. Available online: http://www.ecs.umass.edu/biofuels/
Images/Roadmap2-08.pdf (accessed on 15 January 2021).
49. Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J.
Mol. Sci. 2008, 9, 1621–1651. [CrossRef]
50. Song, H.; Huh, Y.S.; Lee, S.Y.; Hong,W.H.; Hong, Y.K. Recovery of succinic acid produced by fermentation of a metabolically
engineered Mannheimia succiniciproducens strain. J. Biotechnol. 2007, 132, 445–452. [CrossRef]
51. Li, Q.; Wang, D.; Hu, G.; Xing, J.; Su, Z. Integrated bioprocess for high-efficiency production of succinic acid in an expanded-bed
adsorption system. Biochem. Eng. J. 2011, 56, 150–157. [CrossRef]
52. Davison, B.H.; Nghiem, N.P.; Richardson, G.L. Succinic Acid Adsorption from Fermentation Broth and Regeneration. Appl.
Biochem. Biotechnol. 2004, 114, 653–670. [CrossRef]
53. López-Garzón, C.S.; Ottens, M.; van derWielen, L.A.; Straathof, A.J. Direct downstream catalysis: From succinate to its diethyl
ester without intermediate acidification. Chem. Eng. J. 2012, 200–202, 637–644. [CrossRef]
Sustainability 2021, 13, 6794 27 of 30
54. Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products.
Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [CrossRef]
55. Fu, L.; Gao, X.; Yang, Y.; Aiyong, F.; Hao, H.; Gao, C. Preparation of succinic acid using bipolar membrane electrodialysis. Sep.
Purif. Technol. 2014, 127, 212–218. [CrossRef]
56. Jansen, M.L.A.; van Gulik,W.M. Towards large scale fermentative production of succinic acid. Curr. Opin. Biotechnol. 2014, 30,
190–197. [CrossRef] [PubMed]
57. Salles, I.M.; Dorotyn, S.; Soucaile, P. A new process for the continuous production of succinic acid from glucose at high yield, titer,
and productivity. Biotechnol. Bioeng. 2008, 99, 129–135. [CrossRef]
58. Luque, R.; Lin, C.S.K.; Du, C.; MacQuarrie, D.J.; Koutinas, A.; Wang, R.; Webb, C.; Clark, J.H. Chemical transformations of
succinic acid recovered from fermentation broths by a novel direct vacuum distillation-crystallisation method. Green Chem. 2009,
11, 193–200. [CrossRef]
59. Huang, H.-J.; Ramaswamy, S.; Tschirner, U.; Ramarao, B. A review of separation technologies in current and future biorefineries.
Sep. Purif. Technol. 2008, 62, 1–21. [CrossRef]
60. Datta, D.; Kumar, S.; Uslu, H. Status of the Reactive Extraction as a Method of Separation. J. Chem. 2015, 2015, 1–16. [CrossRef]
61. John, J.J.; Kuhn, S.; Braeken, L.; Van Gerven, T. Ultrasound assisted liquid–liquid extraction with a novel interval-contact reactor.
Chem. Eng. Process. Process. Intensif. 2017, 113, 35–41. [CrossRef]
62. Moraes, L.D.S.; Kronemberger, F.D.A.; Ferraz, H.C.; Habert, A.C. Liquid–liquid extraction of succinic acid using a hollow fiber
membrane contactor. J. Ind. Eng. Chem. 2015, 21, 206–211. [CrossRef]
63. King, C.J.; Dtarr, J. Recovery of Carboxylic Acids fromWater by Precipitation from Organic Solutions. U.S. Patent 5,104,492, 14
April 1992.
64. King, C.J.; Poole, L.J. Craboxylic Acid Sorption Regeneration Process. U.S. Patent 5,412,126, 2 May 1995.
65. Sprakel, L.M.J.; Holtkamp, A.F.M.; .Bassa, R.; .Schuur, B. Swing processes for solvent regeneration in liquid-liquid extraction of
succinic acid. Chem. Eng. Process. Process Intensif. 2019, 143, 107600. [CrossRef]
66. Sprakel, L.M.J.; Schuur, B. Review: Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif.
Technol. 2019, 211, 935–957. [CrossRef]
67. Bayazit, S.S.; Uslu, H.; Inci, I.S. Comparison of the efficiencies of amine extractants on lactic acid with different organic solvents. J.
Chem. Eng. Data 2009, 56, 750–756. [CrossRef]
68. Djas, M.; Henczka, M. Reactive extraction of citric acid using supercritical carbon dioxide. J. Supercrit. Fluids 2016, 117, 59–63.
[CrossRef]
69. Jipa, I.; Dobre, T.; Stroescu, M.; Stoica, A. Acetic acid extraction from fermentation broth: Experimental and modelling studies.
Rev. Chim. 2009, 60, 1084–1089.
70. Chen, L.J.; Zeng, A.; Dong, H.B.; Li, Q.; Niu, C.C. A novel process for recovery and refining of L-lactic acid from fermentation
broth. Bioresour. Technol. 2012, 112, 280–284. [CrossRef]
71. Lateef, H.; Gooding, A.; Grimes, S. Use of 1-hexyl-3- methylimidazolium bromide ionic liquid in the recovery of lactic acid from
wine. J. Chem. Technol. Biotechnol. 2012, 87, 1066–1073. [CrossRef]
72. Wang, J.J.; Pei, Y.C.; Zhao, Y.; Hu, Z.G. Recovery of amino acids by imidazolium based ionic liquids from aqueous media. Green
Chem. 2005, 7, 196–202. [CrossRef]
73. Mikkola, J.P.; Virtanen, P.; Sjöholm, R. Aliquat 336®—a versatile and affordable cation source for an entirely new family of
hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [CrossRef]
74. Marták, J.; Schlosser, Š. Extraction of lactic acid by phosphonium ionic liquids. Sep. Purif. Technol. 2007, 57, 483–494. [CrossRef]
75. Oliveira, F.S.; Araújo, J.M.; Ferreira, R.; Rebelo, L.P.N.; Marrucho, I.M. Extraction of L-lactic, L-malic, and succinic acids using
phosphonium-based ionic liquids. Sep. Purif. Technol. 2012, 85, 137–146. [CrossRef]
76. Lee, S.C.; Kim, H.C. Batch and continuous separation of acetic acid from succinic acid in a feed solution with high concentrations
of carboxylic acids by emulsion liquid membranes. J. Memb. Sci. 2011, 367, 190–196. [CrossRef]
77. Yedur, S.; Berglung, K.S.; Dunuwila, D.D. Succinic Acid Production and Purification. U.S. Patent 6,265,190, 24 July 2001.
78. Berglund, K.A.; Yedur, S.; Dunuwila, D. Succinic Acid Production and Purification. U.S. Patent 5,958,744, 28 September 1999.
79. Eder, R.J.P.; Schmitt, E.K.; Grill, J.; Radl, S.; Woelfler, G.H.; Khinast, J.G. Seed loading effects on the mean crystal size of
acetylsalicylic acid in a continuous-flow crystallization device. Cryst. Res. Technol. 2011, 46, 227–237. [CrossRef]
80. Liu, Z.Z.; Ma, C.Y.; Hu, Y.D.; Wang, X.Z. Effect of seed loading and cooling rate on crystal size and shape distributions in protein
crystallization—A study using morphological population balance simulation. Comput. Chem. Eng. 2010, 34, 1945–1952. [CrossRef]
81. Hojjati, H.; Rohani, S. Cooling and seeding effect on supersaturation and final crystal size distribution (CSD) of ammonium
sulphate in a batch crystallizer. Chem. Eng. Process. 2005, 44, 949–957. [CrossRef]
82. Doki, N.; Seki, H.; Takano, K.; Asatani, H.; Yokota, A.M.; Kubota, N. Process control of seeded batch cooling crystallization of the
metastable -form glycine using an in-situ ATR-FTIR spectrometer and an in-situ FBRM particle counter. Cryst. Growth Des. 2004,
4, 949–953. [CrossRef]
83. Pratiwi, A.I.; Matsumoto, M.; Kondo, K. Permeation of succinic acid through ionic liquid membrane. J. Chem. Eng. Jpn. 2013, 46,
383–388. [CrossRef]
84. Thuy, N.T.H.; Boontawan, A. Production of very-high purity succinic acid from fermentation broth using microfiltration and
nanofiltration-assisted crystallization. J. Membr. Sci. 2017, 524, 470–481. [CrossRef]
Sustainability 2021, 13, 6794 28 of 30
85. Glassner, D.A.; Elankovan, P.; Beacom, D.; Berglund, R. Purification process for succinic acid produced by fermentation. Appl.
Biochem. Biotechnol. 1995, 51–52, 73–82. [CrossRef]
86. Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of
industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [CrossRef]
87. Yoon, Y.; Leuptow, R.M. Removal of organic compound by RO and NF membranes. J. Membr. Sci. 2005, 261, 76–86. [CrossRef]
88. Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-art and
recent developments. J. Membr. Sci. 2007, 288, 1–12. [CrossRef]
89. Xu, F.; Sun, J.; Konda, N.V.S.N.M.; Shi, J.; Dutta, T.; Scown, C.D.; Simmons, B.A.; Singh, S. Transforming biomass conversion
with ionic liquids: Process intensification and the development of a high-gravity, one-pot process for the production of cellulosic
ethanol. Energy Environ. Sci. 2015, 9, 1042–1049. [CrossRef]
90. Wang, Y.; Zhang, N.; Huang, C.; Xu, T. Production of monoprotic, diprotic, and triprotic organic acids by using electrodialysis
with bipolar membranes: Effect of cell configurations. J. Membr. Sci. 2011, 385–386, 226–233. [CrossRef]
91. Saremirad, P.; Gomaa, H.; Zhu, J. Effect of flow oscillations on mass transfer in electrodialysis with bipolar membrane. J. Membr.
Sci. 2012, 405–406, 158–166. [CrossRef]
92. McKinlay, J.B.; Vieille, C.; Zeikus, J.G. Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol. 2007, 76, 727–740.
[CrossRef]
93. Jaquet, A.; Quan, L.; Marison, I.; Vonstockar, U. Factors influencing the potential use of Aliquat 336 for the in situ extraction of
carboxylic acids from cultures of Pseudomonas putida. J. Biotechnol. 1999, 68, 185–196. [CrossRef]
94. Arola, K.; Van Der Bruggen, B.; Mänttäri, M.; Kallioinen, M. Treatment options for nanofiltration and reverse osmosis concentrates
from municipal wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2049–2116. [CrossRef]
95. Prochaska, K.; Antczak, J.; Regel-Rosocka, M.; Szczygiełda, M. Removal of succinic acid from fermentation broth by multistage
process (membrane separation and reactive extraction). Sep. Purif. Technol. 2018, 192, 360–368. [CrossRef]
96. Bonnélye, V.; Guey, L.; Del Castillo, J. UF/MF as RO pre-treatment: The real benefit. Desalination 2008, 222, 59–65. [CrossRef]
97. Wang, C.; Li, Q.; Tang, H.; Yan, D.; Zhou,W.; Xing, J.;Wan, Y. Membrane fouling mechanism in ultrafiltration of succinic acid
fermentation broth. Bioresour. Technol. 2012, 116, 366–371. [CrossRef]
98. Juang, R.S.; Chen, H.L.; Lin, Y.C. Ultrafiltration of coagulation-pretreated Serratia marcescens fermentation broth: Flux characteristics
and prodigiosin recovery. Sep. Sci. Technol. 2012, 47, 37–41. [CrossRef]
99. Cho, Y.H.; Lee, H.D.; Park, H.B. Integrated Membrane Processes for Separation and Purification of Organic Acid from a Biomass
Fermentation Process. Ind. Eng. Chem. Res. 2012, 51, 10207–10219. [CrossRef]
100. Nigam, M.O.; Bansal, B.; Chen, X.D. Fouling and cleaning of whey protein concentrate fouled ultrafiltration membranes.
Desalination 2008, 218, 313–322. [CrossRef]
101. AbdEl-Salam, M.H. Membrane techniques: Application of reverse osmosis. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.;
Academic Press: Cambridge, MA, USA, 2003.
102. Pandey, S.R.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary
effluent: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 125–145. [CrossRef]
103. Ricci, B.C.; Ferreira, C.D.; Marques, L.S.; Martins, S.S.; Reis, B.G.; Amaral, M.C. Assessment of the chemical stability of
nanofiltration and reverse osmosis membranes employed in treatment of acid gold mining effluent. Sep. Purif. Technol. 2017, 174,
301–311. [CrossRef]
104. Phanthumchinda, N.; Rampai, T.; Prasirtsak, B.; Thitiprasert, S.; Tanasupawat, S.; Assabumrungrat, S.; Thongchul, N. Alternative
reverse osmosis to purify lactic acid from a fermentation broth. Chem. Ind. Chem. Eng. Q. 2018, 24, 179–190. [CrossRef]
105. Diltz, R.A.; Marolla, T.V.; Henley, M.V.; Li, L. Reverse osmosis processing of organic model compounds and fermentation broths.
Bioresour. Technol. 2007, 98, 686–695. [CrossRef]
106. Duranceau, S.J. Membrane Practices for Water Treatment; American Water Works Association: Denver, CO, USA, 2001; pp. 59–62.
107. Paranjape, S.; Reardon, R.; Foussereau, X. Pretreatment technology for reverse osmosis membrane used in wastewater reclamation
application past, present and future a literature review. Proc. Water Environ. Fed. 2003, 459–486. [CrossRef]
108. Hoek, E.M.V.; Allred, J.; Knoell, T.; Jeong, B.H. Modeling the effects of fouling on full-scale reverse osmosis processes. J. Membr.
Sci. 2008, 314, 33–49. [CrossRef]
109. Koyuncu, I.; Sengur, R.; Truken, T.; Guclu, S.; Pasaoglu, M.E. Advances in water treatment by microfiltration, ultrafiltration and
nanofiltration. In Advances in Membrane Technologies for Waste Water Treatment; Woodhead Publishing: Cambridge, UK, 2015; pp.
83–128. [CrossRef]
110. Law, J.Y.; Mohammed, A. Separation of succinate from organic acid salts using nano filtration membranes. Chem. Eng. Trans.
2017, 56, 1705–1710. [CrossRef]
111. Kang, S.H.; Chang, Y.K. Removal of organic acid salts from simulated fermentation broth containing succinate by nanofiltration.
J. Membr. Sci. 2005, 246, 49–57. [CrossRef]
112. Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Jahim, J.M. New polymeric membrane nanofiltration for succinate recovery: A
comparative study. J. Polym. Res. 2017, 24, 197. [CrossRef]
113. Antczak, J.; Szczygiełda, M.; Prochaska, K. Nanofiltration separation of succinic acid from post-fermentation broth: Impact of
process conditions and fouling analysis. J. Ind. Eng. Chem. 2019, 77, 253–261. [CrossRef]
Sustainability 2021, 13, 6794 29 of 30
114. Choi, J.-H.; Fukushi, K.; Yamamoto, K. A study on the removal of organic acids from wastewaters using nanofiltration membranes.
Sep. Purif. Technol. 2008, 59, 17–25. [CrossRef]
115. Luo, J.;Wan, Y. Effects of pH and salt on nanofiltration—a critical review. J. Membr. Sci. 2013, 438, 18–28. [CrossRef]
116. Zaman, N.K.; Malaysia, U.K.; Yih, L.J.; Vun, C.P.; Rohani, R.; Mohammad, A.W. Recovery of Organic Acids from Fermentation
Broth Using Nanofiltration Technologies: A Review. J. Phys. Sci. 2017, 28, 85–109. [CrossRef]
117. Tan, J.P.; Jahim, J.M.; Harun, S.; Wu, T.Y.; Mumtaz, T. Utilization of oil palm fronds as a sustainable carbon source in biorefineries.
Int. J. Hydrog. Energy 2016, 41, 4896–4906. [CrossRef]
118. Zaman, N.K.; Rohani, R.; Mohammad, A.W. Polyimide membranes for organic salts recovery from model biomass fermentation.
Malays. J. Anal. Sci. 2016, 20, 1481–1490. [CrossRef]
119. Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Isloor, M.; Md, J.; Zaman, N.K.; Jamaliah, M.J. Investigation of Succinic Acid
Recovery from Aqueous Solution and Fermentation Broth using Polyimide Nanofiltration Membrane. J. Environ. Chem. Eng.
2017, 1–36. [CrossRef]
120. Jusoh, N.; Othman, N.; Nasruddin, N.A. Emulsion liquid membrane technology in organic acid purification. Malays. J. Anal. Sci.
2016, 20, 436–443. [CrossRef]
121. Jusoh, N.; Othman, N. Stability of Palm Oil-based Emulsion Liquid Membrane for Succinic Acid Extraction from Aqueous
Solution. J. Appl. Membr. Sci. Technol. 2016, 19, 1–17. [CrossRef]
122. Jusoh, N.; Noah, N.F.M.; Othman, N. Extraction and Recovery Optimization of Succinic Acid Using Green Emulsion Liquid
Membrane Containing Palm Oil as the Diluent. Environ. Prog. Sustain. Energy. 2018, 38. [CrossRef]
123. Othman, N.; Jusoh, N.; Mohar, M.; Muhammad, B.R.; Norul, F.; Mohamed, N. Extraction of succinic acid from real fermentation
broth using emulsion liquid membrane process. Malays. J. Anal. Sci. 2018, 22, 1090–1101. [CrossRef]
124. Werner, S.; Haumann, M.;Wassersheid, P. Ionic liquids in chemical engineering. Annu Rev. Chem. Biomol. Eng. 2010, 1, 203–230.
[CrossRef]
125. Pratiwi, A.I.; Matsumoto, M. Chapter 5—Separation of Organic Acids through Liquid Membranes Containing Ionic Liquids. In
Ionic Liquids in Separation Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 189–206. [CrossRef]
126. Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and
future potential. J. Membr. Sci. 2011, 370, 1–22. [CrossRef]
127. Wang, X.; Chang, V.W.; Tang, C.Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation:
Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [CrossRef]
128. Haupt, A.; Lerch, A. Forward Osmosis Application in Manufacturing Industries: A Short Review. Membranes 2018, 8, 47.
[CrossRef] [PubMed]
129. Azlan, N.M.; Peshev, D.; Livingston, A. Energy consumption for desalination—A comparison of forward osmosis with reverse
osmosis, and the potential for perfect membranes. Desalination 2016, 377, 138–151. [CrossRef]
130. Sreedhar, I.; Khaitan, S.; Gupta, R.; Reddy, B.M.; Venugopal, A. An odyssey of process and engineering trends in forward osmosis.
Environ. Sci. Water Res. Technol. 2018, 4, 129–168. [CrossRef]
131. Abou El-Nour, F.H. Water Desalination Studies Using Forward Osmosis Technology, a Review. Arab. J. Nucl. Sci. Appl. 2016, 49,
167–176.
132. Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A
review. Water Res. 2014, 58, 179–197. [CrossRef]
133. Munirasu, S.; Haija, M.A.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review.
Process Saf. Environ. Prot. 2016, 100, 183–202. [CrossRef]
134. Law, J.Y.; Mohammad, A.W. Osmotic concentration of succinic acid by forward osmosis: Influence of feed solution pH and
evaluation of seawater as draw solution. Chin. J. Chem. Eng. 2018, 26, 976–983. [CrossRef]
135. Garcia-Aguirre, J.; Alvarado-Morales, M.; Fotidis, I.A.; Angelidaki, I. Up-concentration of succinic acid, lactic acid, and ethanol
fermentations broths by forward osmosis. Biochem. Eng. J. 2019. [CrossRef]
136. Lee, H.D.; Lee, M.Y.; Hwang, Y.S.; Cho, Y.H.; Kim, H.W.; Park, H.B. Separation and Purification of Lactic Acid from Fermentation
Broth Using Membrane-Integrated Separation Processes. Ind. Eng. Chem. Res. 2017, 56, 8301–8310. [CrossRef]
137. Sun, Y.; Yan, L.; Fu, H.; Xiu, Z. Salting-out extraction and crystallization of succinic acid from fermentation broths. Process Biochem.
2014, 49, 506–511. [CrossRef]
138. Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends
Biotechnol. 2008, 26, 100–108. [CrossRef] [PubMed]
139. Lin, S.K.C.; Du, C.; Blaga, A.C.; Camarut, M.; Webb, C.; Stevens, C.V.; Soetaert, W. Novel resin-based vacuum distillationcrystallisation
method for recovery of succinic acid crystals from fermentation broths. Green Chem. 2010, 12, 666–671. [CrossRef]
140. Thuy, N.T.H.; Kongkaew, A.; Flood, A.; Boontawan, A. Fermentation and crystallization of succinic acid from Actinobacillus
succinogenes ATCC55618 using fresh cassava root as the main substrate. Bioresour. Technol. 2017, 233, 342–352. [CrossRef]
141. Wang, C.; Ming, W.; Yan, D.; Zhang, C.; Yang, M.; Liu, Y.; Zhang, Y.; Guo, B.; Wan, Y.; Xing, J. Novel membrane-based
biotechnological alternative process for succinic acid production and chemical synthesis of bio-based poly (butylene succinate).
Bioresour. Technol. 2014, 156, 6–13. [CrossRef]
142. Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997, 56, 1–24. [CrossRef]
Sustainability 2021, 13, 6794 30 of 30
143. Weatherley, L.R.; Gangu, A.S.; Scurto, A.M.; Petera, J. Chapter 12: Process Intensification of Enzymatic Biotransformation
Processes. In Intensification of Biobased Processes; Royal Society of Chemistry: London, UK, 2018; pp. 268–288.
144. Shi, J.; Gladden, J.M.; Sathitsuksanoh, N.; Kambam, P.; Sandoval, L.; Mitra, D.; Zhang, S.; George, A.; Singer, S.W.; Simmons, B.A.; et al. One-pot ionic liquid pretreatment and saccharification of switchgrass. Green Chem. 2013, 15, 2579–2589. [CrossRef]
145. Fischer, S.; Thummler, K.; Pfeiffer, K.; Liebert, T.; and Heinze, T. Evaluation of molten inorganic salt hydrates as reaction medium for the derivatization of cellulose. Cellulose 2002, 9, 293–300. [CrossRef]
146. De, S.; Dutta, S.; Saha, B. One-pot conversions of lignocellulosic and algal biomass into liquid fuels. Chem Sus. Chem. 2012, 5,1826–1833. [CrossRef]
147. Field, R. Membranes for Water Treatment; Wiley: Hoboken, NJ, USA, 2010.
148. Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A review of membrane fouling and its control in algal-related membrane processes. Bioresour. Technol. 2018, 264, 343–358. [CrossRef]
149. Munshi, F.M.; Church, J.; McLean, R.; Maier, N.; Anwar Sadmani, A.H.M.; Duranceau, S.J.; Lee, W.H. Dewatering algae using an aquaporin-based d polyethersulfone forward osmosis membrane. Separ. Purif. Technol. 2018, 204, 154–161. [CrossRef]
150. Song, W.; Ravindran, V.; Koel, B.E.; Pirbazari, M. Nanofiltration of natural organic matter with H2O2/UV pretreatment: Fouling mitigation and membrane surface characterization. J. Membr. Sci. 2004, 241, 143–160. [CrossRef]
151. Sun, F.; Lu, D.; Ho, J.S.; Chong, T.H.; Zhou, Y. Mitigation of membrane fouling in a seawater-driven forward osmosis system for waste activated sludge thickening. J. Clean. Prod. 2019, 241, 118373. [CrossRef]
152. Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [CrossRef]
153. Gao, Y.; Qin, J.; Wang, Z.; Østerhus, S.W. Backpulsing technology applied in MF and UF processes for membrane fouling mitigation: A review. J. Membr. Sci. 2019, 587, 117136. [CrossRef]
154. Zhang, Y.; Fu, Q. Algal fouling of microfiltration and ultrafiltration membranes and control strategies: A review. Sep. Purif. Technol. 2018, 203, 193–208. [CrossRef]
155. Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The use of ultrasound to mitigate membrane fouling in desalination and water treatment. Desalination 2018, 443, 143–164. [CrossRef]
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998.
- Chen, H.; Wang, L. Chapter 1-Introduction. In Technologies for Biochemical Conversion of Biomass; Academic Press: Cambridge, MA, USA, 2017; pp. 1–10.
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11.
- Xie, H.; Gathergood, N. The Role of Green Chemistry in Biomass Processing and Conversion; John Wiley & Sons: Toronto, ON, Canada, 2012; pp. 1–496. ISBN 978-0-470-64410-2. Available online: https://www.wiley.com/en-us/9780470644102 (accessed on 18 May 2021).
- Bernick, L. The Right Chemistry. The $100 Billion Business Case for Safer Chemistry. Available online: https://www.greenbiz.com/article/100-billion-business-case-safer-chemistry (accessed on 31 October 2020).
- Deselnicu, D.C.; Militaru, G.; Deselnicu, V.; Zăinescu, G.; Albu, L. Towards a circular economy—A zero waste programme for Europe. In Proceedings of the CAMS 2018—7th International Conference on Advanced Materials and Systems, Timisoara, Romania, 28–31 March 2018; pp. 563–568.
- Molino, A.; Casella, P.; Marino, T.; Iovine, A.; Dimatteo, S.; Balducchi, R.; Musmarra, D. Succinic Acid Production as Main Player of the Green Chemistry Industry by using Actinobacillussuccinogens. Chem. Eng. Trans. 2020, 79, 289–294.
- Cespi, D.; Esposito, I.; Cucciniello, R.; Anastas, P.T. Beyond the beaker: Benign by design society. Curr. Res. Green Sustain. Chem. 2020, 3, 100028.
- Morales, M.; Ataman, M.; Badr, S.; Linster, S.; Kourlimpinis, I.; Papadokonstantakis, S.; Hatzimanikatis, V.; Hungerbühler, K. Sustainability assessment of succinic acid production technologies from biomass using metabolic engineering. Energy Environ. Sci. 2016, 9, 2669–2926.
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and applications of succinic acid. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630.
- Erickson, B.; Nelson Winters, P. Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol. J. 2012, 7, 176–185.
- Law, J.Y.; Mohammad, A.W.; Tee, Z.K.; Zaman, N.K.; Md Jahim, J.; Santanaraj, J.; Sajab, M.S. Recovery of succinic acid from fermentation broth by forward osmosis-assisted crystallization process. J. Membr. Sci. 2019, 583, 139–151.
- Transparency Market Research. The Succinic Acid Market. pp. 1–95. Available online: https://www.transparencymarketresearch.com/succinic-acid.html (accessed on 13 April 2021).
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66.
- Sekoai, P.T.; Awosusi, A.A.; Yoro, K.O.; Singo, M.; Oloye, O.; Ayeni, A.O.; Bodunrin, M.; Daramola, M.O. Microbial cell immobilization in biohydrogen production: A short overview. Crit. Rev. Biotechnol. 2018, 38, 157–171.
- Apergis, N.; Payne, J.E. Renewable energy, output, CO2 emissions, and fossil fuel prices in Central America: Evidence from a nonlinear panel smooth transition vector error correction model. Energy Econ. 2014, 42, 226–232.
- Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.E.; Al-Shannag, M. Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314.
- Kumar, R.; Basak, B.; Jeon, B.-H. Sustainable production and purification of succinic acid: A review of membrane-integrated green approach. J. Clean. Prod. 2020, 277, 123954–123973.
- Kurzrock, T.; Weuster-Botz, D. Recovery of succinic acid from fermentation broth. Biotechnol. Lett. 2010, 32, 331–339.
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod. Bioref. 2014, 8, 16–29.
- Zheng, P.; Fang, L.; Xu, Y.; Dong, J.J.; Ni, Y.; Sun, Z.H. Succinic acid production from corn stover by simultaneous saccharification and fermentation using Actinobacillus succinogenes. Bioresour. Technol. 2010, 101, 7889–7894.
- Willke, T.; Vorlop, K.D. Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl. Microbiol. Biotechnol. 2004, 66, 131–142.
- Beauprez, J.J.; De Mey, M.; Soetaert, W.K. Microbial succinic acid production: Natural versus metabolic engineered producers. Process Biochem. 2010, 45, 1103–1114.
- Li, Q.; Wang, D.; Wu, Y.; Li, W.; Zhang, Y.; Xing, J.; Su, Z. One step recovery of succinic acid from fermentation broths by crystallization. Sep. Purif. Technol. 2010, 72, 294–300.
- Cimini, D.; Argenzio, O.; Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of succinic acid from Basfia succiniciproducens up to the pilot scale from Arundo donax hydrolysate. Bioresour. Technol. 2016, 222, 355–360.
- Kurzrock, T.; Weuster-Botz, D. New reactive extraction systems for separation of bio-succinic acid. Bioprocess Biosyst. Eng. 2011, 34, 779–787.
- Alexandri, M.; Vlysidis, A.; Papapostolou, H.; Tverezovskaya, O.; Tverezovskiy, V.; Kookos, I.K.; Koutinas, A. Downstream separation and purification of succinic acid from fermentation broths using spent sulphite liquor as feedstock. Separ. Purif. Technol. 2019, 209, 666–675.
- Pal, P.; Kumar, R.; Chakravarthi, D.V.; Chakrabortty, S. Modelling and simulation of continuous production of L (+) glutamic acid in a membrane-integrated bioreactor. Biochem. Eng. J. 2016, 106, 68–86.
- Kumar, R.; Pal, P. Fermentative production of poly (g-glutamic acid) from renewable carbon source and downstream purification through a continuous membrane-integrated hybrid process. Bioresour. Technol. 2015, 177, 141–148.
- Sosa, P.A.; Roca, C.; Velizarov, S. Membrane assisted recovery and purification of bio-based succinic acid for improved process sustainability. J. Membr. Sci. 2016, 501236–501247.
- Londono, A.O. Separation of Succinic Acid from Fermentation Broths and Esterification by a Reactive Distillation Method. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2010. Available online: https://d.lib.msu.edu/etd/888. (accessed on 13 July 2020).
- Awosusi, A.A. Bioconversion of Waste Lignocellulosic Biomass (South African corn cob) to Succinic Acid in Molten Hydrate Solvent System. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2018. Available online: https://hdl.handle.net/10539/25888 (accessed on 24 October 2020).
- Cho, Y.H.; Lee, H.D.; Park, H.B. Integrated Membrane Processes for Separation and Purification of Organic Acid from a Biomass Fermentation Process. Ind. Eng. Chem. Res. 2012, 51, 10207–10219.
- Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997, 56, 1–24.
- Weatherley, L.R.; Gangu, A.S.; Scurto, A.M.; Petera, J. Chapter 12: Process Intensification of Enzymatic Biotransformation Processes. In Intensification of Biobased Processes; Royal Society of Chemistry: London, UK, 2018; pp. 268–288.
- Xu, F.; Sun, J.; Konda, N.V.S.N.M.; Shi, J.; Dutta, T.; Scown, C.D.; Simmons, B.A.; Singh, S. Transforming biomass conversion with ionic liquids: Process intensification and the development of a high-gravity, one-pot process for the production of cellulosic ethanol. Energy Environ. Sci. 2015, 9, 1042–1049.
- Shi, J.; Gladden, J.M.; Sathitsuksanoh, N.; Kambam, P.; Sandoval, L.; Mitra, D.; Zhang, S.; George, A.; Singer, S.W.; Simmons, B.A.; et al. One-pot ionic liquid pretreatment and saccharification of switchgrass. Green Chem. 2013, 15, 2579–2589.
- Fischer, S.; Thummler, K.; Pfeiffer, K.; Liebert, T.; and Heinze, T. Evaluation of molten inorganic salt hydrates as reaction medium for the derivatization of cellulose. Cellulose 2002, 9, 293–300.
- De, S.; Dutta, S.; Saha, B. One-pot conversions of lignocellulosic and algal biomass into liquid fuels. Chem Sus. Chem. 2012, 5, 1826–1833.
