Rapid developments in the field of plant genome editing using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) systems necessitate more detailed consideration of the delivery of the CRISPR system into plants. Successful and safe editing of plant genomes is partly based on efficient delivery of the CRISPR system. Along with the use of plasmids and viral vectors as cargo material for genome editing, non-viral vectors have also been considered for delivery purposes.
- genome editing
- CRISPR
- nanoparticles
- exosomes and liposomes
1. Introduction
Genetic engineering of plants is at the core of environmental sustainability efforts, natural product synthesis for pharmaceuticals, and agricultural crop engineering and can help meet the needs of the growing human population under changing global climatic conditions [1][2][1,2]. Genetic transformation allows the transfer of a foreign gene of interest (a transgene), encoding a trait into the plant cell and introduce the desired trait into a crop. For more success in the genetic engineering of plants, new methods are needed to allow passive transport of diverse biomolecules into a larger range of plant species. Gene vectors, constructed to transfer a gene of interest into a host cell, play an important role in genetic transformations [3].
Nanotechnology-based biotransformation is relatively efficient; however, its combination with commonly applied approaches in transgenic plant development could improve transformation efficiency, productivity, and the chances of minimizing transgene silencing. Nanoparticle-mediated gene transfer depends not only on the defined nanoparticle size and shape but also on the surface functionalization of nanoparticles, nucleic acid protection ability, and biocompatibility [4].
Different forms of magnetic nanoparticles and carbon nanotubes have been used for the development of a sustainable plant transformation method. Various nanostructures hold impressive possibilities for the improvement of our expertise and methodology and the enhancement of plant genetic transformation, specifically in molecular plant-breeding programs [5][6][7][5,6,7]. Modification of physicochemical properties of nanocarriers, protoplast, and plasma membrane could significantly improve liposomal- and nanoparticle-encapsulated gene delivery into the plant cell and help to establish gene transfer methods as a sole process rather than adjuvant [3].
For transfection, a number of nanoparticle-based delivery cargos are available, as opposed to the few methods of gene transformation in plants. For the transformation of plastids or mitochondria especially, which is not possible through agrobacterium-mediated transformation, other vector delivery methods are required. Nanoparticle-based delivery could be successful in bridging the species limitation in plants. Moreover, in the future, new delivery tools could also help carry and transform molecules with a higher molecular weight i.e., proteins. Furthermore, production of non-GMOs to address regulatory concerns would also be a tremendous future achievement. Nanomaterial-based cell membrane vectors can deliver drugs while keeping their configuration and folding in their native states and maintaining stability [8].
2. Reagents in CRISPR/Cas
CRISPR/Cas reagents can be inserted into cells as mRNA, DNA, or protein and any of these formats can be employed for electroporation, transfection, and microinjection. In this section, the three reagents of CRISPR/Cas, i.e., RNA, DNA and protein, are discussed.
These differences give Cas12a some benefit over Cas9, i.e., Cas12a small crRNA are suitable for multiplexed genome editing, since more of them can be bundled in one vector than Cas9 sgRNA [9][23]. In addition, the sticky 5′ overhang left by Cas12a can also be utilized for DNA assembly that is far more target-specific than conventional restriction enzyme cloning. Ultimately, Cas12a cleaves 18–23 base pairs of DNA downstream from the PAM site.
Cas13 is an RNA-guided endonuclease, which implies that it does not cut DNA but rather single-stranded RNA [10][24]. Cas13 is directed to the ssRNA target by its crRNA and binds and cleaves the target. Similar to Cas12a, Cas13 stays attached to the target and later non-discriminately cleaves other ssRNA molecules. This collateral cleavage characteristic has been used for the production of various diagnostic technologies.
However, some researchers also use guide RNAs with different crRNA and tracrRNA components, which are usually referred to as two-piece gRNAs or simply as cr: tracrRNAs (pronounced as CRISPR tracer RNAs). Synthetic cr:tracrRNA kits are also available for this purpose ( https://www.synthego.com/products/crispr-kits/crrna-tracrrna accessed on 3 February 2021). A number of software tools are available to design an optimal guide RNA sequence with minimum off-target effects and maximum on-target efficiency. They include tools such as Desktop Genetics ( https://www.crunchbase.com/organization/desktop-genetics accessed on 3 February 2021) , Synthego Design Tool ( https://www.synthego.com/products/bioinformatics/crispr-design-tool accessed on 3 February 2021), and Benchling ( https://www.benchling.com/ accessed on 3 February 2021).
3. Nanoparticle-Based Carriers in CRISPR/Cas
Successful reports of nanoparticle-based delivery of CRISPR/Cas.
Interestingly, recently reported artificial multi-layer NPs exhibited considerable delivery efficiency with CRISPR/Cas9 systems, demonstrating the superiorities of multi-layer structures in delivering CRISPR cargos [11][19]. The multi-layer structures of these NPs are quite similar to viral vectors; for example, they have (1) a cationic core (organic or inorganic) to stabilize the CRISPR cargos; (2) an organic shell (mainly a lipid layer) to protect the inner complexes; and (3) multifunctional ligands anchored on shell to facilitate the bio-interactions with host cells.
CRISPR cargos can be efficiently and stably encapsulated into the multi-layer NPs, and the material compositions can significantly influence the extra-/intracellular fate of these cargos and thereby the genome-editing efficiency. To transport CRISPR tools into the nucleus so that they can exert their biological functions, an ideal non-viral vector needs to load the cargos inside of cells efficiently, accurately deliver the cargos to the specific site, and further mediate the efficient cellular endocytosis of cargos.
By coating the nanoclew with polymer polyethylenimine (PEI) to induce endosomal escape, the group demonstrated roughly 36% efficiency in the delivery of CRISPR/Cas9:RNP with the nanoclew (compared with 5% with bare Cas9:sgRNA and PEI). This allowed the nanoclew to attain efficiencies comparable to other high-efficiency CRISPR/Cas9 delivery systems but still contain no viral components (or, indeed, any exogenous material besides repeating DNA and PEI) [12][96].
4. Emerging Delivery Tools for CRISPR Cargos in Plants
There are various emerging tools for delivery of CRISPR cargos in plants. There are three basic CRISPR reagents that have been frequently used for genome editing ( Figure 13 ). Nanotechnology is advancing with each passing day; moreover, the other related technologies are achieving perfection in the delivery of CRISPR cargos. One such example is pollen magnetofection-mediated genome editing. Magnetofection is a genetic transformation technique that uses magnetic force to absorb a vector connected with magnetic nanoparticles (MNPs). This approach uses positively charged Fe 3O 4-coated polyethylenemine MNPs and negatively charged vectors to form MNP-DNA complexes. Furthermore, the pollens are combined with the complexes and the magnetic field is implemented and applied for pollination. This technology has been applied effectively for cotton.
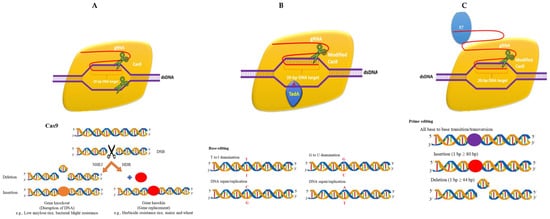
Currently, two approaches are commonly used for distributing CRISPR/Cas9 components: the first uses CRISPR/Cas9 vectors, and the second one uses a vector-less CRISPR/Cas9 method. Magnetofection is based on vector-free editing which is useful for the generation of non-transgenic crops.
To date, there has been no research on the editing of genome-mediated pollen magnetofection. However, the benefit of this approach is that it makes it possible to directly transfer the CRISPR/Cas9 ribonucleoproteins to pollen. This technique can reduce the time needed for tissue culture and transgenic organism selection.
