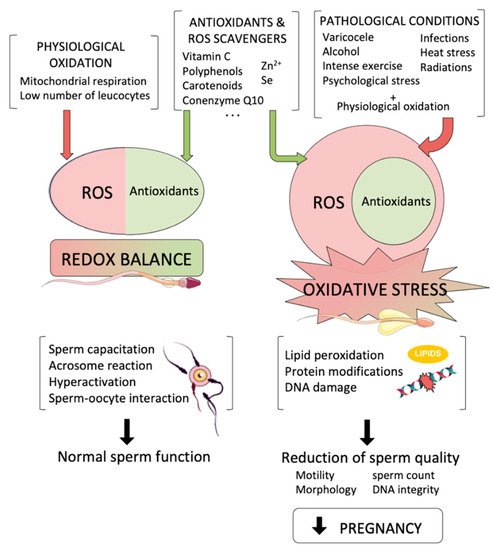
The spermatozoon is a highly specialized cell, whose main function is the transport of the intact male genetic material into the oocyte. During its formation and transit throughout male and female reproductive tracts, sperm cells are internally and externally surrounded by reactive oxygen species (ROS), which are produced from both endogenous and exogenous sources. While low amounts of ROS are known to be necessary for crucial physiological sperm processes, such as acrosome reaction and sperm–oocyte interaction, high levels of those species underlie misbalanced antioxidant-oxidant molecules, generating oxidative stress (OS), which is one of the most damaging factors that affect sperm function and lower male fertility potential.
1. Introduction
Human infertility is recognized as a disease by the World Health Organization (WHO) and has nowadays an estimated prevalence of 7–15%, with 50–80 million people being affected around the world
[1][2][1,2]. These high ranks of incidence exemplify the differences between developed and developing countries, the latter being the ones with higher rates, a fact that has been associated to lower familiar income and less health-care accessibility
[3][4][3,4]. Despite these worrying numbers, mounting evidence from surveys and comparative studies suggest that infertility grows year over year
[4][5][4,5]. In this scenario, and despite the high relevance given to the female factor in the nineties, it is now well accepted that dysfunctions can also affect spermatozoa, so that a male factor is thought to be present in half of the infertile couples, being the only or main cause of infertility in about 20–30% of cases
[6][7][8][6,7,8]. For this reason, research focused on the male factor has been increasing over the last decades, revealing new knowledge about causes and treatments of infertility. Although significant advances on understanding the etiology of male infertility have been made, little is still known about the underlying physiopathology in a high number of particular cases. In most couples, attributing a single cause to infertility is difficult since there is usually a multifactorial, sometimes unknown, pathology. Recent research has come up with different indicators and biomarkers that are able to explain male infertility, such as some specific related genes, concrete epigenetic profiles, and several mRNAs that are responsible for poor sperm quality and/or embryo development
[9][10][11][9,10,11]. On the other hand, anatomical affectations such as vas deferens agenesia, hormonal issues causing azoospermia, or varicocele are also factors that help diagnose infertility in the male
[12][13][12,13]. Moreover, increasing male age, environmental pollution, diet, heat stress, and obesity are also known to be causes for a reduction of sperm quality. In these latter ones, however, it is difficult to establish their precise incidence in the physiopathology of a single male, despite the efforts in defining ranks or risk areas for each parameter. In a scenario where a human male is exposed to some of these exogenous affectations, an increase in oxidative stress is usually observed
[14]. An excess of reactive oxygen species (ROS) can cause unbalance with the antioxidant capacity, reaching pathological levels and originating fertility issues. Infertility caused by oxidative stress has been widely studied for years, and multiple studies analyzing its clinical effect have been performed, in relation to both natural fertility and assisted reproductive techniques.
2. Sources of Oxidative Stress with Potential Effects to the Male Germline
Oxidative stress is related to the disbalance of oxidant molecules in cells. Reactive oxygen species refer to oxygen-derivate molecules that are highly reactive due to their free electrons or radicals. These group of molecules, which have a half-life of nanoseconds, include superoxide (·O
2−), hydrogen peroxide (H
2O
2), proxyl radical (·R OO), or hydroxyl (·OH
−). Less common but also present in sperm cells are the reactive nitrogen species (RNS), which include nitric oxide (·NO), dinitrogen trioxide (N
2O
3), and peroxinitrite (ONOO-). The presence of oxidative stress affecting sperm function has been consistently reported since the late forties and early fifties, when different authors pointed out that hydrogen peroxide exerted a detrimental impact on sperm
[15]. Nowadays, this damage is irrefutable and, over the last decades, multiple investigations have been oriented towards identifying the possible sources of ROS in order to understand their action mechanism and their consequences. This knowledge has allowed the discovery of new treatment options for human patients, thus improving their reproductive chances. Regarding the male germline, reactive oxygen species can be produced either endogenous or exogenous. From here on, we review these two ways of producing ROS that, despite being necessary at low levels, contribute to a pathological state at high levels.
2.1. Endogenous Sources of ROS
One should note that the spermatozoon itself is a source of ROS due to its metabolic activity, and that other immature sperm cells present in the semen are also important sources of free radicals. While disbalances of ROS have disruptive effects that will be described below in other sections, low levels of ROS are known to be necessary for the sperm cell to perform natural functions. For instance, nitric oxide and hydrogen peroxide are necessary compounds to achieve capacitation, enabling acrosome reaction, which is controlled by reactive oxygen species
[16][17][16,17]. Moreover, sperm hyperactivation or its interaction with the oocyte is mediated by ROS, thereby being also essential for achieving their fertilizing ability
[18][19][18,19] (
Figure 1). For these reasons, after spermatogenesis and epididymal maturation sperm cells have low levels of oxidative radicals.
Figure 1. Reactive oxygen species (ROS) are by-products that are necessary for essential sperm functions. On the left, a redox balance is achieved when physiological oxidation is compensated by antioxidants, so that physiological oxidation allows sperm to perform their normal functions. However, when pathological conditions lead to an increase in intracellular ROS levels (right), oxidative stress affects sperm cells causing a reduction in sperm quality that leads to a reduction in pregnancy achievement.
Endogenous free radicals can be generated as a by-product of cell metabolism, or by enzymatic activity. Sperm metabolism is a major source of ROS (
Figure 1). The activity of mitochondria, which are located in the mid-piece, is, together with glycolysis, essential for sperm motility and capacitation. These mitochondria generate ATP through respiratory electron chain and oxidative phosphorylation, which are based on transferring electrons from inner mitochondrial membrane complexes to oxygen and on pumping of protons to the intermembrane space. These protons are ultimately used to synthesize ATP via complex V (ATP synthase). In this scenario, ROS are by-products of the electron chain activity and are especially generated at complexes I and III, which mainly release superoxide and hydroxyl radicals into the matrix and the intermembrane space
[20]. These free radicals are then converted into hydrogen peroxide by superoxide dismutase.
During spermatogenesis, release of sperm with retained cytoplasm usually occurs. Retention of cytoplasm leads to non-functional and immature sperm which retain glucose-6-phosphate dehydrogenase (G6PDH) that enables the production of intracellular β-nicotinamide adenine dinucleotide phosphate (NADPH). This intracellular NADPH is processed via NADPH oxidase which regenerates NADPH to NADP converting O
2 to superoxide, which is converted to hydrogen peroxide at the intermembrane space by the superoxide dismutase
[21][22][21,22].
The hydrogen peroxide generated through these two different ways is scavenged by glutatione peroxidase or glutathione-s-reductase, which use reduced glutathione (GSH) as an electrone donor. Reduced glutathione is maintained by an ATP-consuming process, through glutathione synthetase (GSS) or glutathione reductase (GSR), which transforms oxidized glutathione (GSSG) into reduced glutathione in a NADPH dependent way
[23][24][23,24].
2.2. Exogenous Sources of ROS
2.2.1. Varicocele
Varicocele is a dilatation of the pampiniform plexus of the spermatic cord that is considered the most common correctable cause of male infertility. The incidence of varicocele in the general population is about 15%, and different studies have determined that its incidence among infertile men is about 35–44%, these figures increasing up to 45–81% in the case of secondary infertility
[12]. The dilatation of varicose veins coupled to insufficient venous valves cause a blood reflux and an increase in the blood pressure to the vein wall; this increases testis temperature, which usually has to be 2 °C lower than that of the body. These two varicocele consequences lead to an increase of reactive oxygen species and a reduction of antioxidant capacity
[25] (
Figure 1). Varicoceles are divided into different grades (subclinical and clinical, with grades 1, 2, or 3) according to their clinical features. It is known that their effect is higher as grade increases, and several studies have pointed out that varicocelectomy, the most common procedure for varicocele correction, is an effective method to reduce sperm OS and sperm DNA damage in clinical varicoceles
[26][27][26,27].
2.2.2. Infections and Leucocytospermia
Immune response against infections cause inflammation of tissues to promote leucocyte infiltration. Leukocytes are an important source of oxidative stress, and it has been described that a single leukocyte produces 1000 times more ROS than a single spermatozoon, via increasing NADPH production
[28] (
Figure 1). Although every ejaculate contains a certain number of leukocytes, leucocytospermia consists of an increase in that number and usually results from infections
[29]. While the increase in ROS has been reported to affect both leukospermic and non-leukospermic patients, it is apparent that the extent at which patients with augmented leukocyte counts suffer from higher oxidative stress is higher
[30]. This increment has been demonstrated to be associated to a detrimental impact on different conventional semen parameters, such as motility, morphology, and concentration
[31][32][33][31,32,33].
2.2.3. Alcohol and Tobacco
It is well known that consumption of alcohol, tobacco, and different recreational drugs contribute to serious, negative effects on the organism. Regarding alcohol, a study in rats concluded that continued alcohol intake causes a decrease in testicular reduced glutathione concentration, a decrease in testicular superoxide dismutase activity, an increase in testicular malondialdehyde concentration, and an increase in sperm DNA damage. In addition, fertility rates of male rats ingesting alcohol were demonstrated to be lower than those of the control group
[34]. Other studies reported similar results, describing detrimental effects on mitochondria, with a significant increase in ROS generation, and observing epigenetic modifications in the germline
[35][36][35,36]. In addition, different studies have shown that chronic ethanol intake has been related to a decrease of cell proliferation in testes; to the induction of testicular apoptosis, increasing the Fas ligand and upregulating p53 gene expression; and to epididymal damage
[37][38][39][37,38,39]. A deregulation of the apoptotic response at testicular level has been described as an issue caused by oxidative stress, and named as abortive apoptosis
[40][41][40,41]. Moreover, autophagy can be also activated as a protective role to cooperate with apoptosis during spermatogenesis
[42][43][42,43]
On the other hand, tobacco smokers are exposed to thousands of chemicals, which are demonstrated to be carcinogenic and the cause of several diseases that may lead to death. Most of these chemicals are demonstrated to increase free radicals and ROS coupled to a reduction of antioxidant activity, which leads to a higher rate of sperm DNA fragmentation and loss of sperm motility
[44][45][44,45] (
Figure 1).
2.2.4. Physical Exercise and Heat Stress
Exercise is beneficial for different aspects of health. In the case of reproductive physiology, while improved fertility potential has been identified in animals exposed to regular exercise
[46] and sedentary men have been reported to present worse sperm quality
[47][48][47,48], other studies have shown that exercise has no improving effect on conventional spermiogram parameters
[49]. In addition, intense physical exercise or cycling is considered to be detrimental for fertility, and both an increase in oxidative stress and a reduction in sperm motility were observed in studies focusing on these types of exercise
[50][51][50,51]. This increment in oxidative stress and the reduction in sperm quality could also be related to an increase in testicular temperature (
Figure 1). Scrotal temperature is known to be 2 °C lower than that the body core temperature, and increases in this value have been shown to impair sperm quality. In fact, it has been reported that every 1 °C of increase correlates to a 14% drop in sperm production
[52]. In the same way, some reports have shown that habits such as prolonged car sitting, taking regular sauna bath, or wearing tight fitting underwear can have an impact on testis heat stress, reducing sperm quality
[53].
2.2.5. Radiations and Pollution
Radiations can be divided into ionizing and non-ionizing. Non-ionizing radiation is the most used by human beings, and one can find in this category from cell phones, which use extremely low frequency, to microwave ovens and radars, which are in the radio frequency range
[54]. The effect of non-ionizing radiations produced by mobile phones, microwaves or WIFI devices to sperm oxidative stress and its fertilizing ability is a topic of increasing interest among the scientific community. Different multiple in vitro and in vivo studies aiming at addressing this issue by testing sperm from animal models and human beings have shown that mobile phone’s radiation causes an increase in reactive oxygen species that induces lipid peroxidation and a decline in the antioxidant capacity, induced, amongst others, by the decrease in reduced glutathione levels
[55][56][57][58][55,56,57,58]. Moreover, other studies have associated laptop computer WIFI to increases of DNA damage and decreases of sperm parameters, such as motility, count, and morphology
[59][60][59,60]. In summary, although non-ionizing radiations are not able to cause DNA alterations directly, they have an indirect potential of affecting fertility through increasing pro-oxidant molecules (
Figure 1).
X-rays, γ-rays, and α-particles are ionizing radiations, which are rather more dangerous than the non-ionizing ones at different health levels. Exposure of cells to ionizing radiations increase ROS generation and induce their senescence
[61]. Radiation induces direct DNA breaks and potentially affects proteins and membranes through increased ROS levels (
Figure 1). In fact, serious health problems, such as different types of cancer, can arise from the exposure to ionizing radiation; in this context, it is worth noting that, due to their lack of antioxidant defense, sperm cells are especially vulnerable
[62].
Environmental pollution has been found to increase ROS generation and lead to a reduction in sperm quality. For instance, on the one hand and according to studies analyzing semen from human males exposed to traffic pollution, car smoke pollutants potentially reduce men fertility
[63] by affecting membrane lipids, generating DNA damage, and even changing expression patterns of proteins involved in spermatogenesis
[64][65][66][64,65,66]. On the other hand, continuous exposure to phthalate derivatives have been correlated to increases in reactive oxygen species and decreases in enzymatic and non-enzymatic antioxidants in animal models
[67], and in the seminal plasma of infertile patients involved in assisted reproductive programs
[68]. While these are only two examples of how air pollutants may affect men fertility, strong evidence is still required to support the urgent need of reducing environmental toxicants to improve reproductive efficiency
[69].