Domesticated crops suffer from major genetic bottlenecks while wild relatives retain higher genomic diversity. Wild soybean (Glycine soja Sieb. & Zucc.) is the presumed ancestor of cultivated soybean (Glycine max [L.] Merr.), and is an important genetic resource for soybean improvement. Among the East Asian habitats of wild soybean (China, Japan, Korea, and Northeastern Russia), the Korean peninsula is of great importance based on archaeological records, domestication history, and higher diversity of wild soybeans in the region. The collection and conservation of these wild soybean germplasms should be put on high priority. Chung’s Wild Legume Germplasm Collection maintains more than 10,000 legume accessions with an intensive and prioritized wild soybean germplasm collection (>6000 accessions) guided by the international code of conduct for plant germplasm collection and transfer. The center holds a library of unique wild soybean germplasms collected from East Asian wild habitats including the Korean mainland and nearby islands. The collection has revealed interesting and useful morphological, biochemical, and genetic diversity. This resource could be utilized efficiently in ongoing soybean improvement programs across the globe.
- Glycine soja
- Korean wild soybean
- in situ conservation
- germplasm conservation
- wild legumes
- genetic diversity
- crop wild relatives
1. Introduction
2. Distribution and Conservation of Wild Soybean in Korea
2.1. Geographical Distribution of Wild Soybean in Korea
2.2. Archaeological Records and Background
3. Collection of Wild Soybean Germplasm in Korea
| Instcode | Institute | No. of Accessions | % | WS | LR | BL | AC | OT |
|---|---|---|---|---|---|---|---|---|
| CHN001 | ICGR-CAAS | 32,021 | 14 | 21 | 79 | |||
| USA033 | SOY | 21,075 | 9 | 10 | 80 | 5 | 4 | 1 |
| KOR011 | RDAGB-GRD | 17,644 | 8 | <1 | 45 | 5 | 1 | 50 |
| TWN001 | AVRDC | 15,314 | 7 | <1 | <1 | 100 | ||
| BRA014 | CNPSO | 11,800 | 5 | 100 | ||||
| JPN003 | NIAS | 11,473 | 5 | 5 | 33 | 21 | 40 | |
| RUS001 | VIR | |||||||
| AMFO | ||||||||
| 1582 | ||||||||
| 1 | ||||||||
| 100 | ||||||||
| THA005 | FCRI-DA/TH | 1510 | 1 | 100 | ||||
| MEX001 | INIA-Iguala | 1500 | 1 | 100 | ||||
| Corporación Colombiana de Investigación Agropecuaria Tibaitata (Colombia) | ||||||||
| IFVCNS | ||||||||
| Institute for Field and Vegetable Crops (Serbia) | ||||||||
| ICCPT Fundul | ||||||||
| Research Institute for Cereals and Technical Plants Fundulea (Romania) | ||||||||
| Species | No. of Accessions |
|---|---|
| Glycine max (L.) Merr | 600 |
| Glycine soja | 6232 |
| Amphicarpaea edgeworthii | 1300 |
| Vigna vexillata | 810 |
| Rhynchosia volubilis | 225 |
| Trait | Gene/QTL | Chromosome/Locus Group | Source (accession) | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield Related Traits | |||||||||||||
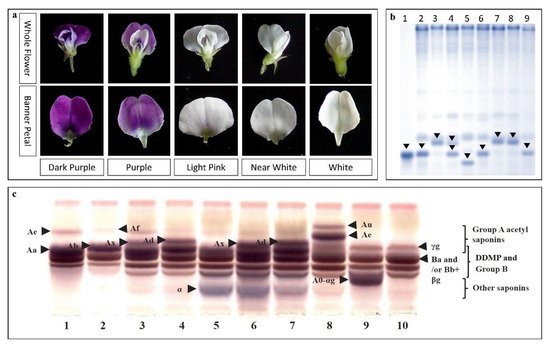
| Flower color | Dihydroflavonol-4-reductase 4 (w4-s1 allele) (DFR) | B2 (Ch 14) | WS (CW13133) | [46] | |||||||||
| Flavonoid 3′5′-hydroxylase (F3′5′H) and DFR | B2 (Ch 14) | WS (CW12700 and CW13381) | [45] | ||||||||||
| DFR2 (w4-s1) | B2 (Ch 14) | WS (CW13133) × CS (IT182932) | [47] | ||||||||||
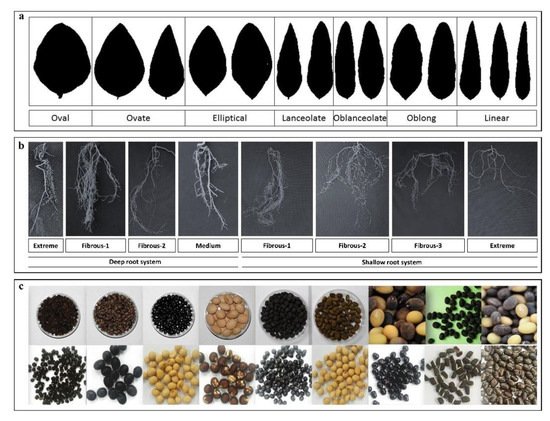
| Oil and local breeding related traits | QTLs | 3, 8, 13, 17, | WS × CS | [17] | Phaseolus nipponensis | 700 | |||||||
| Shoot fresh weight | QTLs | 6, 15, 19 | WS (PI 483463) × CS (Hutcheson) | [48] | Other wild legumes Dunbaria villosa6439 |
3 | 9 | 40 | 41 | (Thunb.) Makino Vigna umbellate11 |
|||
| (Thumb.) Ohwi & Ohashi Vigna angularis var. nipponensis (ohwi) ohwi & ohashi Vigna angularis (Willd.) Ohwi & Ohashi |
700 | ||||||||||||
| Biological nitrogen fixation related traits | QTLs | 7,8,12,17,18 | WS (W05) × CS (C08) | [49] | IND016 | AICRP-Soybean | 4022 | 2 | <1 | 100 | |||
| Small seed, Yellow seed color, Soy sprout |
CIV005 | ||||||||||||
| Total | 10,567 | WS (PI135624) | [50] | IDESSA | 3727 | 2 | |||||||
| High yield | 100 | ||||||||||||
| B2 (U26) | WS (PI 407305) | [51] | TWN006 | TARI | 2745 | 1 | 100 | ||||||
| Yield, height and maturity | QTLs | C2, E, K and M | WS × CS | [52] | DEU146 | ||||||||
| Seed yield, 100-seed weight, seed filling period, maturity, height, lodgingIPK | QTLs2661 | 1 | 1 | 23 | 53 | A1, J, N, H, F, L | WS × CS | [23 | 53] | ||||
| ZWE003 | CBICAU | ||||||||||||
| 2236 | 1 | QTLs | Multiple | CS (NN86-4) × WS (PI342618B) | [100 | ||||||||
| 54 | ] | IDN182 | ICRR | 2198 | 1 | <1 | 100 | ||||||
| QTLs | 1, 2, 6, 8, 13, 14, 17, 20 | CS (Williams 82) × WS (PI 366121) | [55] | AUS048 | ATCFC | 2121 | 1 | 3 | <1 | 38 | 52 | 6 | |
| Biotic Stress Tolerance | NGA039 | IITA | 1909 | 1 | 5 | 4 | 1 | 90 | |||||
| Soybean cyst nematode resistance | Rhg1, Rhg4 locus and QTLs | G, A2, B1 | CS (Magellan) × WS (PI 404198A) | [56] | FRA060 | ||||||||
| Glyma18g196200, Glyma18g077900, Glyma18g078000, Glyma18g106800, Glyma18g107000, Glyma18g107100 | Ch18 | WS | [57] | ||||||||||
| Glyma18g106800, Glyma18g064100 | |||||||||||||
| QTLs (cqSCN-006, cqSCN-007) | I, J, K, O | WS (PI 468916, 464925B) | [58][59] | PHL130 | IPB-UPLB | 1381 | 1 | 100 | |||||
| Markers (A245_1 and Satt598) | 15, 18 | WS (PI 468916) | [60] | UKR001 | IR | 1288 | 1 | 3 | 1 | 21 | 72 | 3 | |
| COL017 | ICA/REGION 1 | 1235 | 1 | <1 | 64 | 13 | |||||||
| Resistance to southern root knot nematode | WS | [61]22 | |||||||||||
| Foxglove aphid resistance (Aulacorthum solani) |
Raso2 | 7 | CS (Williams 82) × CS (PI 366121) | [62] | SRB002 | IFVCNS | 1200 | 1 | 100 | ||||
| Aphid resistance (Aphis glycines) | Rag3c and Rag6 | 8, 16 | WS (85-32) | [63] | ROM002 | ICCPT Fundul | 1024 | <1 | |||||
| QTLs | 62 | 38 | 8, 16, | <1 | |||||||||
| WS | WS (PI 518282) |
[64] [65] |
Others (166) | 81,839 | 36 | 11 | 4 | 27 | 51 | ||||
| Sclerotinia stem rot | QTLs | 3, 8, 20 | WS × CS | [66][67][68] | Total | 229,944 | 100 | ||||||
| Resistance to Mung bean yellow mosaic India virus | Ch 8 and 146 | WS (PI 393551) | [69]17 | 7 | 13 | 56 | |||||||
| ICGR-CAAS | Institute of Crop Germplasm Resources, Chinese Academy of Agricultural Sciences | ||||||||||||
| Resistance to Phtophthora soja | QTLs | J, I, G (Ch 16, 18, 20) | WS (PI 407162) × CS (V71-370) | [70] | SOY | Soybean Germplasm Collection, United States Department of Agriculture, Agricultural Research Services | |||||||
| Abiotic Stress Tolerance | RDAGB-GRD | ||||||||||||
| Salt tolerance | Genetic Resources Division, National Institute of Agricultural Biotechnology, Rural Development Administration (Korea) | ||||||||||||
| WS (N23232 BB52) | [71] | AVRDC | World Vegetable Centre (former Asian Vegetable Research and Development Centre) | ||||||||||
| Allele (Ncl locus) | WS (PI 483463) | [72] | CNPSO | ||||||||||
| QTLs | Embrapa Soja (Brazil) | ||||||||||||
| D2 (Ch 17) | WS (JWS156-1) | [73 | NIAS | National Institute of Agrobiological Sciences (Japan) | |||||||||
| ] | |||||||||||||
| QTLs | 3 | WS | [74] | VIR | N.I. Vavilov All-Russian Scientific Research Institute of Plant Industry (Russian Federation) | ||||||||
| RLKs CaMs, JA/SA Signaling genes, MAPKs, WRKYs | [67][68] | AICRP-Soybean | All India Coordinated Research Project on Soybean (India) | ||||||||||
| IDESSA | Institut des Savanes (Côte d’Ivoire) | ||||||||||||
| TARI | Taiwan Agricultural Research Institute | ||||||||||||
| IPK | Genebank, Leibniz Institute of Plant Genetics and Crop Plant Research (Germany) | ||||||||||||
| CBICAU | Crop Breeding Institute (Zimbabwe) | ||||||||||||
| ICRR | Indonesian Centre for Rice Research | ||||||||||||
| ATCFC | Australian Tropical Crops & Forages Genetic Resources Centre | ||||||||||||
| IITA | International Institute of Tropical Agriculture | ||||||||||||
| AMFO | G.I.E. Amelioration Fourragère (France) | ||||||||||||
| FCRI-DA/TH | Field Crops Research Institute–Department of Agriculture (Thailand) | ||||||||||||
| INIA-Iguala | Estación de Iguala, Instituto Nacional de Investigaciones Agrícolas (Mexico) | ||||||||||||
| IPB-UPLB | Institute of Plant Breeding, College of Agriculture, University of the Philippines, Los Baños College (Philippines) | ||||||||||||
| IR | Institute of Plant Production n.a. V.Y. Yurjev of UAAS (Ukraine) | ||||||||||||
| ICA/REGION 1 | |||||||||||||
3.1. Chung’s Wild Legume Germplasm Collection
3.2. In situ Conservation of G. soja at CWLGC

| Tolerance to herbicide metribuzin | ||||
| WS (PI 245331, PI 163453) | ||||
| [ | ||||
| 75 | ||||
| ] | ||||
| Root traits | ||||
| QTLs | ||||
| 8, 12 | ||||
| WS (PI 326582A) × CS (PI 552538) | ||||
| [ | ||||
| 76 | ||||
| ] | ||||
| Root architecture | ||||
| Glyma15g42220, Glyma06g46210, Glyma06g45910, Glyma06g45920, Glyma07g32480 | ||||
| 6, 7, 15 | WS (PI 407162) × CS (V71-370) | [77] | ||
| Alkalinity tolerance | ALMT, LEA, ABC transporter, GLR, NRT/POT and SLAH genes | Multiple | WS (N24852) | [78] |
| Drought tolerance | GsWRKY20 | [79] | ||
| Nutrition | ||||
| Seed protein content | Marker pA-245 | C | CS (A81-356022) × WS (PI 468916) | [80] |
| Seed saturated fatty acid contents | SNPs | GS | [81] | |
| Glyma14g121400, Glyma16g068500 | 14, 16 | |||
| Seed unsaturated fatty acids | Glyma16g014000, Glyma07g112100 | 7, 16 | ||
| Linolenic acid | QTLs | Multiple | WS × CS | [82] |
| Pearl Japanese fermented product (Natto) | WS | [61] | ||
| Protein, oil, palmitic acid, stearic acid, oleic acid, linoleic acid, lenolenic acid | QTLs | 2, 7, 14, 16 | WS | [81] |
| Saponin A | Sg-1 | Ch 7 | WS | [65][83] |
| Sg-5 (Glyma15g39090) | Ch 15 | WS × CS | [84][69] | |
| Glyma15g39090 | Ch 15 | WS | [36] | |
| Seed antioxidant, phenolics, and flavonoids | GmMATE1,2,4 | Ch 18,19 | WS (W05) × CS (C08) | [85] |
