Irisin, the circulating peptide originating from fibronectin type III domain-containing protein 5 (FNDC5), is mainly expressed by muscle fibers under peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) control during exercise. In addition to several beneficial effects on health, physical activity positively affects nervous system functioning, particularly the hippocampus, resulting in amelioration of cognition impairments. Recently, FNDC5/irisin detection in hippocampal neurons and the presence of irisin in the cerebrospinal fluid opened a new intriguing chapter in irisin history. Interestingly, in the hippocampus of mice, exercise increases FNDC5 levels and upregulates brain-derived neurotrophic factor (BDNF) expression. BDNF, displaying neuroprotection and anti-inflammatory effects, is mainly produced by microglia and astrocytes.
1. Overview of FNDC5/Irisin System
Irisin is one of the novel exercise-induced myokines firstly described by Boström and colleagues in 2012 [1].
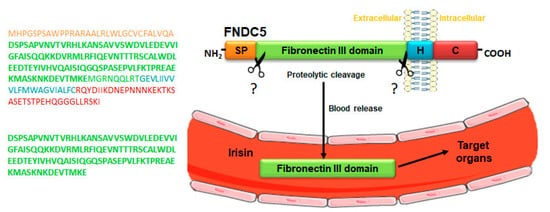
Irisin is produced from a transmembrane glycoprotein, named fibronectin type III domain-containing protein 5 (FNDC5), which is cleaved in its extracellular domain by a yet unidentified enzyme, resulting in the release of a 112 amino acids polypeptide into the bloodstream (
). Interestingly, the discovery of the circulating irisin was subsequent to that of its precursor. In 2002, two different research groups during their cloning studies in mice demonstrated the expression of irisin precursor FNDC5 as peroxisomal protein (PeP) and fibronectin type III repeat-containing protein 2 (FRCP2). In particular, Ferrer-Martínez et al. found PeP in the peroxisome matrix of various cell types, and Teufel et al. discovered the gene encoding FRCP2 while studying different fibronectin type III domains [2][3]. Both of these researchers identified irisin precursor expression in the murine brain. A robust PeP expression at the mRNA level was shown, by Northern blot analyses, only in adult mice brain, while it was absent during mouse development [2]. Conversely, the expression of
). Interestingly, the discovery of the circulating irisin was subsequent to that of its precursor. In 2002, two different research groups during their cloning studies in mice demonstrated the expression of irisin precursor FNDC5 as peroxisomal protein (PeP) and fibronectin type III repeat-containing protein 2 (FRCP2). In particular, Ferrer-Martínez et al. found PeP in the peroxisome matrix of various cell types, and Teufel et al. discovered the gene encoding FRCP2 while studying different fibronectin type III domains [2,3]. Both of these researchers identified irisin precursor expression in the murine brain. A robust PeP expression at the mRNA level was shown, by Northern blot analyses, only in adult mice brain, while it was absent during mouse development [2]. Conversely, the expression of Frcp2
was detected in both embryonic and adult brain tissue, suggesting its specific role during head development and for adult brain functioning [3]. Subsequently, the initial existence of the two different molecules, PeP and FRCP2, gave rise to the recognition of the same product renamed FNDC5. The expression of this membrane glycoprotein is regulated by the transcriptional coactivator peroxisome proliferator-activated receptor gamma co-activator-1α (PGC-1α), which, in turn, is induced by physical activity [1].
Figure 1.
Mechanism of irisin release into the blood. Schematic structure of irisin precursor fibronectin type III domain-containing protein 5 (FNDC5) and its amino acid sequence (upper panel). FNDC5 cleavage, by a yet unknown enzyme at levels of the extracellular domain, gives rise to the release of irisin into the blood, a peptide consisting of a sequence of 112 amino acids (lower panel). Abbreviations: SP, signal peptide; H, hydrophobic domain; C, cytoplasmic domain. Symbols: ? indicates the unknown enzyme.
Comparative studies demonstrated that
Fndc5 is a well-preserved gene among species, and 100% of identity has been found between human and murine irisin sequences [1]. Conversely, only the human FNDC5 gene displays an uncommon ATA start codon [4], and this was considered as a “null mutation” that would prevent human irisin production and its release into the blood [5][6]. In humans, bioinformatics analyses have identified the existence of three isoforms for FNDC5 mRNA that were all expressed during the neural differentiation process [7]. Furthermore, high expression levels of FNDC5 were detected in human fetal brain and spinal cord tissues, suggesting the involvement of this gene in neural tube development [7]. In rodents, FNDC5 expression was revealed in various cerebral regions, including cerebellar Purkinje cells [8], hypothalamus [9], and hippocampus [10].
is a well-preserved gene among species, and 100% of identity has been found between human and murine irisin sequences [1]. Conversely, only the human FNDC5 gene displays an uncommon ATA start codon [4], and this was considered as a “null mutation” that would prevent human irisin production and its release into the blood [5,6]. In humans, bioinformatics analyses have identified the existence of three isoforms for FNDC5 mRNA that were all expressed during the neural differentiation process [7]. Furthermore, high expression levels of FNDC5 were detected in human fetal brain and spinal cord tissues, suggesting the involvement of this gene in neural tube development [7]. In rodents, FNDC5 expression was revealed in various cerebral regions, including cerebellar Purkinje cells [8], hypothalamus [9], and hippocampus [10].
Jedrychowski et al. detected a significant increase of irisin concentration in the plasma of exercised subjects with respect to the sedentary ones (4.3 ng/mL versus 3.6 ng/mL) using a targeted mass spectrometry approach [4]. Besides physical activity, several factors, such as diet, obesity, metabolic diseases, as well as treatments for such conditions, and various other pathological disorders (chronic renal failure, hypothyroidism, musculoskeletal, and neurodegenerative diseases) affect the circulating irisin levels [11][12][13][14]. Very recently, a reduced irisin serum level was found in patients with age-related bone diseases with respect to healthy subjects [15]. By means of ELISA assays and quantitative mass spectrometry analyses, irisin was also found in human cerebrospinal fluid (CSF) [16][17][18]. Recently, Ruan et al. detected the irisin circulating levels in CSF at a concentration of about 0.26–1.86 ng/mL in old male subjects (over 80 years of age) with various diseases [17]. Moreover, an age-related increase in irisin levels in the CSF of healthy humans was found [18].
Jedrychowski et al. detected a significant increase of irisin concentration in the plasma of exercised subjects with respect to the sedentary ones (4.3 ng/mL versus 3.6 ng/mL) using a targeted mass spectrometry approach [4]. Besides physical activity, several factors, such as diet, obesity, metabolic diseases, as well as treatments for such conditions, and various other pathological disorders (chronic renal failure, hypothyroidism, musculoskeletal, and neurodegenerative diseases) affect the circulating irisin levels [11,12,13,14]. Very recently, a reduced irisin serum level was found in patients with age-related bone diseases with respect to healthy subjects [15]. By means of ELISA assays and quantitative mass spectrometry analyses, irisin was also found in human cerebrospinal fluid (CSF) [16,17,18]. Recently, Ruan et al. detected the irisin circulating levels in CSF at a concentration of about 0.26–1.86 ng/mL in old male subjects (over 80 years of age) with various diseases [17]. Moreover, an age-related increase in irisin levels in the CSF of healthy humans was found [18].
In the investigation of irisin tissue targets, Boström et al. were the first to recognize the irisin effect on adipose tissue, showing its ability in inducing white adipose tissue browning (i.e., the transdifferentiation of white adipocytes into brown-like fat cells) via enhancing mitochondrial uncoupling protein 1 (UCP1) expression [1]. Succeeding evidence highlighted more wide-ranging effects of irisin on other tissues as its role in regulating energy metabolism. In particular, the myokine promotes glucose and lipid uptake by skeletal muscles [11][19], increases hepatic glucose and lipid metabolism [20][21], displaying beneficial effects on metabolic disorders, such as diabetes [11][22].
In the investigation of irisin tissue targets, Boström et al. were the first to recognize the irisin effect on adipose tissue, showing its ability in inducing white adipose tissue browning (i.e., the transdifferentiation of white adipocytes into brown-like fat cells) via enhancing mitochondrial uncoupling protein 1 (UCP1) expression [1]. Succeeding evidence highlighted more wide-ranging effects of irisin on other tissues as its role in regulating energy metabolism. In particular, the myokine promotes glucose and lipid uptake by skeletal muscles [11,19], increases hepatic glucose and lipid metabolism [20,21], displaying beneficial effects on metabolic disorders, such as diabetes [11,22].
Irisin displayed a noteworthy anabolic role on bone by the stimulation of osteoblast differentiation and activity [23]. This finding paved the way towards the investigation of irisin effects on hindlimb suspended mice, a widely recognized disuse-induced osteo-sarcopenic model characterized by the reduction of bone and muscle mass. Noticeably, in these mice, irisin positive action was shown on the musculoskeletal system, resulting in the simultaneous restoration of both muscle and bone mass loss [24]. A similar effect on osteoblasts was reported in a 3D in vitro model placed in a microgravity environment [25]. In addition, the last studies evidenced also a new intriguing role of irisin in delaying osteoblast senescence by the downregulation of p21
, a well-known negative cell cycle regulator [15]. Besides osteoblasts, the osteocytes, the most abundant bone cell population, are irisin targets as the myokine improved their functions and displayed anti-apoptotic effects [26].
At present, the complete identification of irisin receptors is still lacking, but Kim et al. proposed integrins, specifically αV/β5, as its receptors on murine adipocytes and osteocytes [27]. However, the binding affinity of irisin to other integrin complexes, as well as the ability of integrin αV/β5 to interact with other ligands, hindered the comprehension of irisin specific effects in vivo.
The identification of the irisin receptor is still a challenge; however, the intracellular signaling pathways activated by irisin have been partially elucidated. Irisin principally exerts its biological functions via mitogen-activated protein kinases (MAPK) signaling; nonetheless, 5′ adenosine monophosphate-activated protein kinase (AMPK), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), and signal transducer and activator of transcription 3 (STAT3)/Snail pathways are also involved in additional effects [28]. In the brain, recent studies demonstrated that the irisin neuroprotective role seems to be mediated by both STAT3 and cyclic adenosine 3′,5′-monophosphate/protein kinase A/cAMP response element-binding protein (cAMP/PKA/CREB) signaling pathways [29][30].
The identification of the irisin receptor is still a challenge; however, the intracellular signaling pathways activated by irisin have been partially elucidated. Irisin principally exerts its biological functions via mitogen-activated protein kinases (MAPK) signaling; nonetheless, 5′ adenosine monophosphate-activated protein kinase (AMPK), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), and signal transducer and activator of transcription 3 (STAT3)/Snail pathways are also involved in additional effects [28]. In the brain, recent studies demonstrated that the irisin neuroprotective role seems to be mediated by both STAT3 and cyclic adenosine 3′,5′-monophosphate/protein kinase A/cAMP response element-binding protein (cAMP/PKA/CREB) signaling pathways [29,30].
Overall, in less than a decade since the discovery of irisin, a large number of studies have revealed that irisin induces a plethora of benefits on human health. In vivo treatment with rec-irisin or the peripheral delivery of FNDC5 with adenoviral vectors evidenced that its beneficial effects recapitulate those of regular exercise on many organs, including brain health, in which the armament of the anti-inflammatory strategy involves the exercise-mimetic hormone irisin [1][10][23][30].
Overall, in less than a decade since the discovery of irisin, a large number of studies have revealed that irisin induces a plethora of benefits on human health. In vivo treatment with rec-irisin or the peripheral delivery of FNDC5 with adenoviral vectors evidenced that its beneficial effects recapitulate those of regular exercise on many organs, including brain health, in which the armament of the anti-inflammatory strategy involves the exercise-mimetic hormone irisin [1,10,23,30].
2. Physical Activity and FNDC5/Irisin in Neuroinflammation
The paradigm that physical activity displays beneficial effects on human health as a non-pharmacological approach is widely recognized. Its benefits are exerted not only on skeletal muscle but even on multiple non-skeletal targets. Indeed, exercise contributes to reducing adiposity and the risk of insulin resistance, displays beneficial effects on cardiovascular diseases, hypertension, bone and joint diseases, and metabolic disorders. Interestingly, exercise increases cerebral blood flow and exerts a plethora of positive effects on the brain, improving cognitive functions, such as memory and attention, and overall neuronal plasticity [31]. These latter effects were mostly detected in elderly subjects and in patients suffering from psychiatric and neurodegenerative diseases. Hippocampus, particularly the dentate gyrus, is the brain region mainly targeted by the positive impact of exercise [10][32][33]. Studies in humans demonstrated an increase in volume and improvement of functional connectivity in the hippocampus of elderly subjects, resulting in memory progress [34]. Studies in mice showed that the beneficial effects of exercise on hippocampal and synaptic function are due to the activation of metastasis-suppressor 1-like (Mtss1L) transcription factor in the dentate gyrus [35].
The paradigm that physical activity displays beneficial effects on human health as a non-pharmacological approach is widely recognized. Its benefits are exerted not only on skeletal muscle but even on multiple non-skeletal targets. Indeed, exercise contributes to reducing adiposity and the risk of insulin resistance, displays beneficial effects on cardiovascular diseases, hypertension, bone and joint diseases, and metabolic disorders. Interestingly, exercise increases cerebral blood flow and exerts a plethora of positive effects on the brain, improving cognitive functions, such as memory and attention, and overall neuronal plasticity [31]. These latter effects were mostly detected in elderly subjects and in patients suffering from psychiatric and neurodegenerative diseases. Hippocampus, particularly the dentate gyrus, is the brain region mainly targeted by the positive impact of exercise [10,32,33]. Studies in humans demonstrated an increase in volume and improvement of functional connectivity in the hippocampus of elderly subjects, resulting in memory progress [34]. Studies in mice showed that the beneficial effects of exercise on hippocampal and synaptic function are due to the activation of metastasis-suppressor 1-like (Mtss1L) transcription factor in the dentate gyrus [35].
Although the mechanism responsible for these beneficial effects are at present not completely elucidated, the molecular mediators in charge seem to be two groups of factors: neurotrophins, produced locally in the brain, and myokines, released by muscle, which can equally or synergistically exert positive responses [36][37].
Although the mechanism responsible for these beneficial effects are at present not completely elucidated, the molecular mediators in charge seem to be two groups of factors: neurotrophins, produced locally in the brain, and myokines, released by muscle, which can equally or synergistically exert positive responses [36,37].
Neurotrophins, a family of proteins crucial for neuron development, growth, and plasticity, represent the supervisors of brain cell health, and their levels are altered in pathological conditions, i.e., neuroinflammation. Neuroinflammation, frequently associated with neurodegenerative diseases, is linked to several factors, such as infections, toxic metabolites, autoimmunity, aging, traumatic brain, or spinal cord injury, etc. [38]. In the presence of these pathological insults, microglia, the main immune cell type of central nervous system (CNS), are the first responders of neuro-inflammatory processes through the activation of phagocytosis and secretion of a variety of soluble factors [39][40]. These resident immune cells, arising from myeloid progenitors in the embryonic yolk sac, express several pattern-recognition receptors (PRRs) that can recognize pathogen-associated molecular patterns (PAMPs) or tissue damage-associated molecular patterns (DAMPs) [39]. Of note, microglia are highly dynamic cells as they can acquire different morphological and functional features. In their steady-state, microglia display a ramified shape with multiple and thin processes by which they constantly monitor the CNS parenchyma [39]. In response to inflammatory stimuli or extended injuries, microglia switch to an amoeboid morphology with enhanced phagocytic activity and pro-inflammatory cytokines production [interleukin (IL)-1 beta (IL-1β), IL-6, and tumor necrosis factor (TNF)α] [39][41]. In addition, microglia also release specific chemokines [42], which are chemoattractants for peripheral immune cells migrating into CNS to cooperate in the resolution of neuroinflammatory response, reactive oxygen species (ROS), and secondary messengers (nitric oxide, NO, and prostaglandins) [38].
Neurotrophins, a family of proteins crucial for neuron development, growth, and plasticity, represent the supervisors of brain cell health, and their levels are altered in pathological conditions, i.e., neuroinflammation. Neuroinflammation, frequently associated with neurodegenerative diseases, is linked to several factors, such as infections, toxic metabolites, autoimmunity, aging, traumatic brain, or spinal cord injury, etc. [38]. In the presence of these pathological insults, microglia, the main immune cell type of central nervous system (CNS), are the first responders of neuro-inflammatory processes through the activation of phagocytosis and secretion of a variety of soluble factors [39,40]. These resident immune cells, arising from myeloid progenitors in the embryonic yolk sac, express several pattern-recognition receptors (PRRs) that can recognize pathogen-associated molecular patterns (PAMPs) or tissue damage-associated molecular patterns (DAMPs) [39]. Of note, microglia are highly dynamic cells as they can acquire different morphological and functional features. In their steady-state, microglia display a ramified shape with multiple and thin processes by which they constantly monitor the CNS parenchyma [39]. In response to inflammatory stimuli or extended injuries, microglia switch to an amoeboid morphology with enhanced phagocytic activity and pro-inflammatory cytokines production [interleukin (IL)-1 beta (IL-1β), IL-6, and tumor necrosis factor (TNF)α] [39,41]. In addition, microglia also release specific chemokines [42], which are chemoattractants for peripheral immune cells migrating into CNS to cooperate in the resolution of neuroinflammatory response, reactive oxygen species (ROS), and secondary messengers (nitric oxide, NO, and prostaglandins) [38].
If microglia activity is impaired, acute or chronic inflammatory processes can persist in the CNS, leading to irreversible outcomes, i.e., neurodegeneration [39].
Besides microglia, other glial cells, such as astrocytes, can contribute to the local immune responses induced by different insults. Astrocytes are the most abundant glial cell population in the adult CNS, controlling the blood–brain barrier integrity, the neuronal function through the production of neurotrophic factors, the extracellular balance of ions, etc. [42]. Similar to microglia, astrocytes are immunocompetent cells as they can take part in the regulation of innate and adaptive immune responses in the injured CNS [43]. Astrocytes express many receptors involved in innate immunity [44], and when activated by inflammatory stimuli, these cells secrete soluble mediators (IL-1β, TNFα, NO, etc.), triggering innate and/or adaptive immune responses [42].
Microglia and astrocytes can strictly cooperate in the inflammatory process. Indeed, the inflammatory factors released by microglia can induce pro-inflammatory astrocyte activation [42][45].
Microglia and astrocytes can strictly cooperate in the inflammatory process. Indeed, the inflammatory factors released by microglia can induce pro-inflammatory astrocyte activation [42,45].
Interestingly, both microglia and astrocytes can display pro-inflammatory (or neurotoxic) and neuroprotective phenotype [42]. According to an early classification, microglia may be divided into M1 (classical activation) and M2 (alternative activation) phenotypes using the same paradigm of macrophage activation [39]. The first corresponds to the above-mentioned pro-inflammatory state that is induced by the concurrent activation of toll-like receptors (TLRs) and interferon-γ (IFN-γ) signaling pathways, while the second represents the anti-inflammatory condition linked to neuroprotection and tissue healing [39]. Astrocytes also may be classified in A1 (pro-inflammatory) and A2 (anti-inflammatory) reactive astrocytes on the basis of their polarization status. However, M1/M2 and/or A1/A2 paradigms are not always acceptable as omic studies have shown multiple activated phenotypes in vivo [39][46].
Interestingly, both microglia and astrocytes can display pro-inflammatory (or neurotoxic) and neuroprotective phenotype [42]. According to an early classification, microglia may be divided into M1 (classical activation) and M2 (alternative activation) phenotypes using the same paradigm of macrophage activation [39]. The first corresponds to the above-mentioned pro-inflammatory state that is induced by the concurrent activation of toll-like receptors (TLRs) and interferon-γ (IFN-γ) signaling pathways, while the second represents the anti-inflammatory condition linked to neuroprotection and tissue healing [39]. Astrocytes also may be classified in A1 (pro-inflammatory) and A2 (anti-inflammatory) reactive astrocytes on the basis of their polarization status. However, M1/M2 and/or A1/A2 paradigms are not always acceptable as omic studies have shown multiple activated phenotypes in vivo [39,46].
The response of microglia and astrocytes to peripheral factors (pro- and anti-inflammatory) proves that there is a clear interaction between the immune system and CNS, and, furthermore, as physical activity has been recently considered an anti-inflammatory agent, exercise, brain, and immune responses are also linked. Indeed, in response to muscle activity, the release of some myokines can affect microglial activation, such as the anti-inflammatory cytokine IL-10, which exerts an inhibitory effect on the microglial activation [47].
IL-6, the principal myokine that appears in the circulation during exercise [48], can display an indirect anti-inflammatory effect through the upregulation of IL-10 expression [49]. Consistent with this finding, in Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) patients, a decrease of IL-6 levels has been detected [50][51]. However, the biological relevance of exercise-derived IL-6 is still hotly debated since it has been demonstrated that its increase is transient and downregulated by constant training [48]. In addition, IL-6 increase is frequently associated with chronic low-grade inflammation [52], and, recently, a longitudinal study proposed IL-6 as a predictor marker of cognitive decline in late midlife [53]. Of note, while other pro-inflammatory cytokines (IL-1 and TNF-α) induce the further expression of inflammatory mediators (NO or matrix metalloproteinases), muscle-derived IL-6 shows a peculiar behavior through its beneficial role on regenerative processes as well as on metabolism regulation [51][54]. Thus, the comprehension of IL-6 effects is still far from being completely elucidated since this myokine can exert a pro-inflammatory or anti-inflammatory role depending on the activation of trans-signaling via its soluble receptor or classic-signaling by its membrane-bound receptor, respectively [51].
IL-6, the principal myokine that appears in the circulation during exercise [48], can display an indirect anti-inflammatory effect through the upregulation of IL-10 expression [49]. Consistent with this finding, in Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) patients, a decrease of IL-6 levels has been detected [50,51]. However, the biological relevance of exercise-derived IL-6 is still hotly debated since it has been demonstrated that its increase is transient and downregulated by constant training [48]. In addition, IL-6 increase is frequently associated with chronic low-grade inflammation [52], and, recently, a longitudinal study proposed IL-6 as a predictor marker of cognitive decline in late midlife [53]. Of note, while other pro-inflammatory cytokines (IL-1 and TNF-α) induce the further expression of inflammatory mediators (NO or matrix metalloproteinases), muscle-derived IL-6 shows a peculiar behavior through its beneficial role on regenerative processes as well as on metabolism regulation [51,54]. Thus, the comprehension of IL-6 effects is still far from being completely elucidated since this myokine can exert a pro-inflammatory or anti-inflammatory role depending on the activation of trans-signaling via its soluble receptor or classic-signaling by its membrane-bound receptor, respectively [51].
A relevant additional role of exercise on neuroinflammation is the ability to upregulate the brain-derived neurotrophic factor (BDNF) levels. Interestingly, BDNF could be the linkage molecule between brain health and exercise, particularly because its production is linked to FNDC5/irisin expression. It has been demonstrated that in the hippocampus of mice, elevated FNDC5 expression was induced by endurance exercise under the control of PGC-1α, and, in this condition, a neuroprotective gene program, including the
Bdnf
gene, was activated [10]. BDNF, mainly released by microglia and astrocytes, plays a pivotal role in neuron formation, survival, and plasticity, contributing to memory and learning. It has been observed that neurons are also a BDNF source [10]. Indeed, forced expression of FNDC5 via adenoviral transfection or exogenous administration of rec-irisin increased Bdnf
gene expression in primary cortical and hippocampal neurons, regardless of glial cells. On the contrary, loss of function of FNDC5 resulted in BDNF reduction [10]. Importantly, it has been shown that BDNF regulated FNDC5 levels according to a homeostatic feedback loop as rec-BDNF administration reduced Fndc5
gene expression, while this effect was attenuated by the BDNF receptor blockage [10].
The anti-inflammatory effect of BDNF through microglia activation may be exerted by means of different pathways. Firstly, it could bind to its receptor tropomyosin receptor kinase B (TrkB), inducing the activation of extracellular-signal-regulated kinase (ERK) and the phosphorylation of CREB, which, in turn, can impede nuclear factor-kappa light chain enhancer of activated B cells (NF-κB) activity, hindering anti-inflammatory gene transcription [55][56]. Moreover, BDNF might act through Akt signaling, which blocks glycogen synthase kinase 3 (GSK-3) activity, thus reducing the activation of NF-κB and inducing CREB activation [57]. Finally, BDNF is also able to control mitogen-activated protein kinase phosphatase 1 (MKP-1), inducing the reduction of p38 and c-Jun N-terminal kinase (JNK) phosphorylation [58][59][60].
The anti-inflammatory effect of BDNF through microglia activation may be exerted by means of different pathways. Firstly, it could bind to its receptor tropomyosin receptor kinase B (TrkB), inducing the activation of extracellular-signal-regulated kinase (ERK) and the phosphorylation of CREB, which, in turn, can impede nuclear factor-kappa light chain enhancer of activated B cells (NF-κB) activity, hindering anti-inflammatory gene transcription [55,56]. Moreover, BDNF might act through Akt signaling, which blocks glycogen synthase kinase 3 (GSK-3) activity, thus reducing the activation of NF-κB and inducing CREB activation [57]. Finally, BDNF is also able to control mitogen-activated protein kinase phosphatase 1 (MKP-1), inducing the reduction of p38 and c-Jun N-terminal kinase (JNK) phosphorylation [58,59,60].
Due to the evidence that the circulating irisin, although with a not yet well-clarified mechanism, can reach the brain and enhance BDNF expression [10], this myokine might be the molecule inducing neuroprotection responses. Consistent with this, in 2017, studies in mice demonstrated that irisin, through the activation of anti-apoptotic signals, as AKT and ERK1/2 pathways, was involved in the neuroprotective effect of physical exercise against cerebral ischemia [61]. In the same year, the irisin engagement in the inhibition of the inflammatory pathway, such as reactive-oxygen species-Nod-like receptor pyrin-3 (ROS-NLRP3) signal, suggesting irisin as a possible treatment in ischemic stroke was shown in neuron cells [62]. In 2018, in vivo findings highlighted that irisin intrathecal injection increased pain threshold [63], and in vitro data showed that irisin supplementation in media collected from astrocytes protected neurons from β-amyloid toxicity [64]. In astrocytes, irisin could also decrease the release of IL-6 and IL-1β cytokines, the expression of cyclooxygenase-2 (COX-2) pro-inflammatory mediator, and phosphorylation of AKT, suggesting an important role of irisin in neurodegenerative diseases [64]. In addition to all this, there are very recent studies that showed a protective role of irisin via the activation of autophagy. Autophagy, as a potent cell strategy for protecting against inflammation, has been proposed as a mechanism by which physical exercise mediates its beneficial effects on health. In accordance with this hypothesis, several reports showed that exercise could trigger autophagy in different tissues and organs [65]. Importantly, in the AD mice model, the genetically-induced hyperactivation of autophagy, by knock-in of a point mutation in the gene Beclin/Becn1
, which is essential in autophagy, resulted in the decrease of amyloid accumulation and limitation of cognitive decline. Likewise, in these mice models, physiological autophagy was induced by physical activity, resulting in protective effects on amyloid accumulation and memory, thereby exerting a protective effect in the brain [66]. In support of autophagy’s importance as an anti-inflammatory strategy and the role of irisin on autophagy, there is the very recent work of Xin and Lu of March 2020 [67]. These authors showed that mitophagy, a peculiar autophagic process that selectively involves mitochondria, was induced by irisin through the upregulation of the expression of optic atrophy 1 ( Opa1
). In particular, irisin activated
Opa1
-induced mitophagy, and this event preserved mitochondrial function, decreased oxidative stress, and inhibited apoptosis of cardiomyocytes following myocardial infarction [67]. Therefore, this irisin shielding mechanism [65] can be added to the other described effects of the myokine, underlying the importance of irisin in alleviating neuroinflammatory responses and neurodegenerative diseases.