There is no solid evidence of vertical transmission of SARS-CoV-2. Reports that have demonstrated in utero infection, among which one demonstrated placental viremia by RT-PCR, in addition to the presence of inflammatory cells in cerebrospinal fluid (CSF), together with neurological manifestations consistent with those described in adult patients, raise concerns
- COVID-19
- fetus
- newborn
- transmission
- immunity
- inflammation
- preterm delivery
- central nervous system
- neurodevelopmental disorders
1. Introduction
Whether it is congenital or not, an infection raises a concern in pregnant women, because it can infect the fetus and increases the risk of teratogenicity. Currently, there is no solid evidence of vertical transmission of SARS-CoV-2. Reports that have demonstrated in utero infection, among which one demonstrated placental viremia by RT-PCR, in addition to the presence of inflammatory cells in cerebrospinal fluid (CSF), together with neurological manifestations consistent with those described in adult patients, raise concerns [1][2][23,65]. Thus, we cannot exclude the possibility of direct viral infection of the central nervous system (CNS) in fetuses.
2. Possible Viral Infection of the Central Nervous System
The CNS is protected against infections thanks to many factors including the several layers of meningeal tissue surrounding the brain, the blood–brain barrier (BBB), and immunosurveillance through the resident immune cells (microglia) and patrolling of memory T cells, which enter through the choroid plexus and migrate within the CSF to then reach the lymph [3][66]. However, pathogens have developed evasion mechanisms to bypass these tight restrictions.
SARS-CoV-2 presents a neurotropism [4][5][67,68] as viral particles have been detected in the brains of deceased COVID-19 patients who developed neurological complications. In a study of 214 patients, Mao et al. found that CNS and peripheral nervous system (PNS) complications occurred in many COVID-19 patients. CNS complications included dizziness, headache, ischemic stroke, and intracranial hemorrhage (Figure 2).
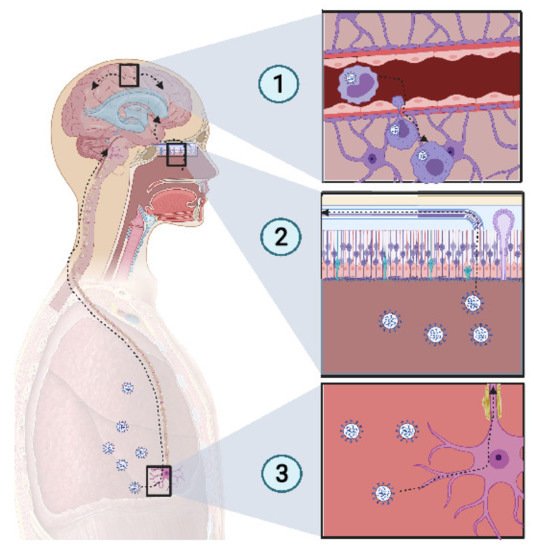
Figure 2. Potential mechanism of CNS invasion by SARS-CoV-2. (1) The blood−brain barrier (BBB) permits a limited exchange, due to the continuous capillary endothelium, with a tight junction supported by a continuous basement membrane and surrounded by astrocyte feet. Monocytes infected with SARS-CoV-2 act as Trojan horses, due to their access to the CNS. Thus, extravasation of infected monocytes can promote CNS infection. (2) The high-spectrum olfactory receptors expressed on the cilia of olfactory neurons may act as receptors for SARS-CoV-2, thus infecting the bipolar neuron in the nasal epithelium. Retrograde movement leads SARS-CoV-2 to the olfactory bulb, which is connected to the limbic system, thus promoting the invasion of the limbic system, including the hippocampus. (3) Chemoreceptive neuron infection in the lower respiratory tract may lead to a retrograde migration of the virus through the nervous network toward the respiratory center in the brain stem, resulting in acute respiratory failure. Created with BioRender.com
2.1. Pathogens Carried by Leukocytes across the BBB
Some bacterial infections, such as Listeria monocytogenes and Burkholderia pseudomallei use CD11+ monocytes in blood as Trojan horses because of their ability to access the CNS [3][66]. SARS-CoV-2 uses CD147, to mediate its infection [6][7][61,69]. It has been reported that SARS-CoV-2 infects tissue-resident CD169+ macrophages [8][70] which express CD147 receptors at their surface, the alternative pathway for SARS-CoV-2 entry. Thus, it is probable that SARS-CoV-2 could similarly invade the CNS by infecting monocytes, enabling access to the tissue.
2.2. Infection through Olfactory Routes
Cilia projecting from olfactory epithelium in the nasal cavity, express a wide variety of receptors that bind a large spectrum of ligands [9][71]. The olfactory nerve expresses a wide range of receptors, thus there is high probability of finding a matching receptor for the virus to invade the CNS through this route. When the virus accesses the olfactory nerve, it enters the olfactory bulb. Several studies have reported a loss of olfactory sense in COVID-19 patients [10][11][72,73]. Because SARS-CoV-2 is present in the nasal environment and has been shown to cause loss of olfaction, it is thought that the virus moves retrogradely from the olfactory bulb to the CNS and then spreads in the brain. The olfactory bulb is part of the limbic system, which connects several brain sections, including the hippocampus. One COVID-19 patient showed a hyperintensity in the right mesial temporal lobe and hippocampus with slight hippocampal atrophy [12][74]. Another recent finding showed that SARS-CoV-2 was detected in the olfactory neurons of some individuals who died from COVID-19, suggesting that the virus could invade the CNS through the olfactory nerve [13][75].
2.3. Retrograde Neuroinvasiveness
Several viruses have been reported to invade the CNS through retrograde movement following an invasion of the CNS. For example, replication of the rabies virus after a canine bite is followed by binding to nicotinic receptors on the motor neuron and moves centripetally without replication until it reaches the spinal cord, where it begins to replicate and to spread rapidly to the brain through retrograde movement [14][76]. SARS-CoV-2 has been reported to infect the respiratory center, where it can be found in high concentrations [15][77]. Previous coronaviruses have been reported to spread via synapse from chemoreceptors and mechanoreceptors in the lower respiratory tract, reaching the cardiorespiratory center [15][16][77,78]. This suggests that the dysfunction of the respiratory center due to potential SARS-CoV-2 damage may play a role in acute respiratory failure in COVID-19 patients. If we link all of these points together, we can suppose that SARS-CoV-2, which inoculates the lower respiratory tract, could infect chemoreceptors and mechanoreceptors in the lung and pass by a retrograde movement to the respiratory center in the brainstem.
2.4. Case Report of Fetal Neuroinvasiveness of SARS-CoV-2
A study made at the University of Paris Saclay, confirmed for the first time transplacental transmission from the mother to a child in the third trimester. A SARS-CoV-2 infected pregnant woman was tested positive in the blood by RT-PCR. She underwent caesarian delivery. Amniotic fluid was collected and tested for SARS-CoV-2 RNA and tests returned positive. In order to confirm vertical transmission, nasopharyngeal and anal swabs were performed in the baby, one hour after delivery and were repeated at day three and day 18, and were positive. Bronchoalveolar lavage was collected in addition to blood sampling for RT-PCR testing and the tests also returned positive. The baby was in a bad condition and presented axial hypertonia and opisthotonos. Magnetic resonance imaging at day 11 showed bilateral gliosis of the deep white periventricular matter. After RNAemia, neurological symptoms translated into increased levels of inflammatory cells in the CSF associated with white matter injury [2][65]. However, this was among the rare cases of vertical transmission reporting with fetal neuroinvasiveness.
3. Cytokine Storm and Inflammation in Neonates?
Cytokine storm is an abnormal and exaggerated immune response that results in excessive inflammation, found in graft-versus-host disease, chimeric antigen receptor T cell, autoimmune diseases, and severe viral infections [17][18][79,80]. SARS-CoV-2 certainly induces an immunopathological response but, above all, causes complications even more severe than the viral infection itself. One major complication of a cytokine storm is acute respiratory distress syndrome (ARDS), which is the main cause of death in COVID-19 patients [19][81]. ARDS is caused by the release of large amount of proinflammatory cytokines by immune and nonimmune cells through the activation of the Nuclear Factor Kappa B (NFκB) pathway. A pathway of NFκB activation in the coronavirus family is through activation of the pattern recognition receptor (PRR) which activates myeloid differentiation primary response 88 (MyD88) which in turn activates NFκB resulting in the production of a cocktail of pro-inflammatory cytokines including tumor necrosis α (TNFα) and IL-6 [20][82]. Virus mediated ACE2 downregulation causes dysregulation in the angiotensin II/angiotensin 1 receptor (AT1R) axis and ACE2/Mas receptor (MasR) axis resulting in the activation of complement subunits C3a and C5a resulting in decreased differentiation of T regulatory cells (Treg) and increased differentiation of T helper 17 (Th17), ultimately resulting in the uncontrolled inflammatory response [21][83]. A 2.9-fold increase in IL-6 concentration has been found in COVID-19 patients, compared with patients with no complications [22][84], and IL-6 has been seen to be higher in nonsurvivors [23][85] (Figure 3).
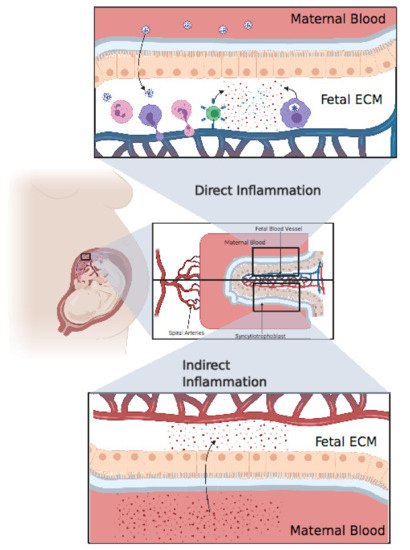
Figure 3. Direct inflammation or indirect inflammation. Direct inflammation: after viral invasion of the placenta, fetal immune cells, including neutrophils, monocytes, and T cells, extravasate from fetal blood to the placental extracellular matter, resulting in an extensive inflammatory response in the fetal extracellular matrix (ECM). Finally, these inflammatory cytokines pass through the blood, resulting in systemic inflammation in the fetus. Indirect inflammation: inflammatory cytokines circulating in maternal blood after a cytokine storm might pass through the placenta, resulting in indirect inflammation. Created with BioRender.com.
These cytokines can come from different sources, namely CD4+ T cells and/or CD14+ CD16+ Monocytes. The cross-talk between innate immune cells and adaptive cells is maintained through these cytokines. Increased expressions of IL-6 and granulocyte monocyte colony stimulating factor (GM-CSF) was detected in COVID-19 patients [24][86]. Other cytokine sources during COVID-19 infection are through pyroptosis (IL6, IL1α, IL1β) [25][15], hemophagocytic lymphohistiocytosis (IFNγ, IL2) [26][27][28][87,88,89], and angiotensin II viral mimicking (IL6 positive feedback loop) [20][82].
Microglia is well known to contribute in neural plasticity, where it acts on sculpturing the neural network and refining neural circuitry, and a dysfunction in microglia may cause microglial neuroplasticity perturbation [29][90]. Microglia expressing ACE2 [30][91] raises thus a concern as SARS-CoV-2 can spread through this axis, leading to neuroplasticity dysfunction or even direct neuroinflammation.
Only a few studies have assessed cytokine production in pregnant women and their neonates. A study showed that pregnant women with SARS-CoV-2 have higher inflammatory cytokine IL-6 levels compared to nonpregnant women [31][92]. Placental inflammation can cause fetal mortality via the release of inflammatory cytokines into fetal blood, resulting in fetal organ failure [32][93]. Baud et al. reported the miscarriage of a fetus in the second trimester from a mother with COVID-19, for which the placental histology revealed subchorionitis infiltrated by neutrophils and monocytes [33][55]. Chen Y et al. reported increased levels of IL-6, two hours after birth in all infants born from mothers infected with SARS-CoV-2. Two of these infants had elevated levels of IgM, but the PCR was negative [34][29]. Dong et al. also reported that a COVID-19 positive mother gave birth to a child with increased IL-6 and IgM above the baseline and a negative PCR [1][23]. Based on these results, we cannot conclude on the presence of fetal infection with SARS-CoV-2 [35][94]. The source of inflammatory cytokines may be direct, if the child is confirmed to be positive for SARS-CoV-2, or indirect through the transcytosis from maternal blood to fetal blood [36][95]. In either case, we must monitor the child’s health because both maternal and fetal inflammation can represent an important environmental risk factor for neurodevelopmental disorders such as schizophrenia, autistic spectrum disorder (ASD), and attention deficit/hyperactivity disorder (ADHD) [37][96]. As mentioned above, it would be very important to consider developing these studies in vivo using appropriate animal models to demonstrate whether SARS-CoV-2 presents a possible threat to the emergence of neurodevelopmental disorders in children exposed to COVID-19 infection.
4. Correlation with Neonate Abnormal Brain Development
Neonates who tested positive for SARS-CoV-2 must be monitored because the virus could have infected their CNS and caused brain damage or disrupted brain circuits, resulting in cognitive challenges or other neurodevelopmental disorders.
From another perspective, the cytokine storm discussed above and reported in neonates could also affect brain development, more specifically in preterm babies [38][97].
Maternal immune activation (MIA) appears to act as a neurodevelopmental disease increasing the risk of neuronal epigenetic modification resulting in neuronal disorders later in life [39][98]. A recent study found that maternal IL-6 was linked with decreased cognition at 12 months old, reinforcing the idea of correlation between maternal inflammation and offspring neuropsychologic disorder [40][99]. Hagberg et al. suggested that, not only does inflammatory reaction in the CNS have the potential to cause acute brain injury, but it also might affect brain development, causing long-term neurological consequences, hypothetically translated by disorders including cognitive impairment, schizophrenia, ASD, multiple sclerosis, cerebral palsy, and Parkinson’s disease [41][100]. It has been noted that during in utero infection, Lipopolysaccharide (LPS) bacteria produce pre-and postnatal brain inflammation, affecting glial cytoarchitecture and amygdala development [42][101]. The Danish newborn screening biobank revealed that a cytokine storm in neonates could increase the risk for ASD [43][102]. Moreover, neonatal encephalopathy is associated with increased levels of IL-6, IL-8, and IL-1β [44][103]. Furthermore, recent studies have shown that placental infection was associated with high levels of IL-6 and IL-8 during parturition, and elevated IL-6, IL-8, IL-1β, and TNFα in the umbilical cord could worsen neurological outcomes after six months [45][104]. Perinatal inflammation during the developmental phase can affect the brain during the fetal period as well as over a long period in postnatal life, where it can affect cortical plasticity and myelination, thus producing an adverse effect on neural connections and the rate of neural message delivery [46][105]. Phosphoinositide 3-kinase δ (PI3Kδ) inhibitors which are used as a therapeutic target for the inhibition of proinflammatory cytokines can be used to suppress a cytokine storm, and consequently might stop the drastic negative effect that inflammation can cause on neonatal brain development [47][106].
