Human dental pulp stem cells (hDPSCs) are some of the most promising stem cell types for regenerative therapies given their high ability differentiate to neural and vascular lineage cells, their growth in animal serum-free media, their secretion of neuroprotective factors and extracellular vesicles, their high resistance to hypoxia/ischemia, their immunomodulatory properties, and their wide range of possibilities to be used in autologous grafts.
- dental pulp stem cells
- neuroregeneration
- neuronal differentiation
- neural markers
- neuroprotection
- immunomodulation
- extracellular vesicles
- tissue engineering
- scaffolds
- cell therapy
1. Introduction: Neural and Mesenchymal Stem Cells, and Neuroregenerative Cell Therapies
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction: Neural and Mesenchymal Stem Cells, and Neuroregenerative Cell Therapies
Neuroregenerative therapies have always been a priority for health research in developed countries due the overwhelming social, economic and dependency burdens suffered by both the affected patients and their close relatives [1][2][3]. The nervous system in humans possesses a very limited capacity of self-repair in the event of injury. This is the reason why nerve lesions caused by trauma or neurodegenerative diseases often result in highly disabling irreversible conditions and chronic dependency [4][5][6]. Unlike other cells of the body, dead or damaged neurons cannot be easily replaced. Neurogenesis takes place in the developing brain, but it declines in the adulthood. Moreover, neuroinflammation and gliosis following neural trauma or disease make the tissue refractory to the rooting and establishment of new neural connections [7][8][9][10].
Neurogenesis is driven by specific multipotent stem cells known as neural stem cells (NSCs), which give rise to both neurons and glial cells. Embryonic NSCs are the first stone paving the way to brain (re)generation and impairments in their correct function are associated to several types of cortical malformations, with dramatic outcomes on the life of an individual [11]. Although the neurogenic capacity is significantly decreased in the adult mammalian brain, two regions bear NSCs during the entire lifetime of different species. The lateral ventricles and the hippocampus harbor adult NSCs capable of triggering a staggered process that ends up in the integration of a newborn neuron into the adult neuronal circuitry [12][13][14]. In humans, adult newborn neurons have been detected in the lateral ventricles, yet in highly infrequent basis [15][16][17]. In the hippocampus, controversial results over the existence of adult neurogenesis have been recently reported and the topic remains currently under hot debate [16][18]. Regardless, there is agreement about the presence of these neurogenic niches during the first years of life in young infants [16][17][19][20], a time period when NSC dysfunctions might play an important role in the development and chronification of neurodegenerative diseases [21]. Indeed, studies in rodents have shown that adult NSCs undergo changes when facing neurodegenerative challenges, including morphological and functional abnormalities that lead to disruption of neurogenesis and contribute to the detrimental tissue environment in these regions [22][23][24]. The existence of NSCs that could react in pathological conditions has been also suggested in other areas, like the cerebral cortex and spinal cord [25][26]. The amygdala, although with rare adult neurogenic events in normal conditions, has also been postulated to bear quiescent NSCs that could get activated upon peripheric lesions, at least in primates [27].
The scarce numbers of adult NSCs or their aberrant alterations in neurodegenerative diseases suppose the lack of a reliable endogenous mechanism to replenish neurons in the event of their loss. Not in vain, stem cells offer the potential to reduce deleterious signaling and improve traumatic lesions [28][29], and also to slowdown the progression of devastating neurodegenerative diseases such as Huntington’s (HD) [30][31], Parkinson’s (PD) [32][33] or Alzheimer’s disease (AD) [34][35][36]. The idea of using stem cells to treat neurodegenerative diseases was proposed very long ago, obtaining valuable and abundant data using fetal human tissue [31][37][38] or induced pluripotent stem cells (IPSCs) [39][40]. However, these methods raise both safety and ethical concerns that are still under intense debate [41][42][43][44][45][46]. The main practical problems are the security, the very low yields of extraction, and the troublesome conditions of intervention on premature infants to harvest human NSCs [34]. Stem cells from the spinal cord of 8-week fetuses have been tested in clinical trials for chronic spinal cord injury [47][48]. However, it is unlikely that these strategies will ever reach a widespread implementation, due to the scarcity of embryo donors and the associated ethical issues. IPSCs have been proposed to overcome ethical concerns about the use of human embryos. IPSCs can be very efficently differentiated to neurons and glial cells [49][50][51] and they have been proposed as a promising alternative for cell therapy in brain and spinal cord injury [39], as a tool to screen genetic bases of neurological diseases [52] or even as an approach to correct alterations in chronic neurodegenerative diseases [53]. However, a better understanding is still required to regulate the generation of specific neuronal and glial populations in a balanced and coordinated manner. Furthermore, the increased risk of cancer related to the use of IPSCs is regarded as a major drawback for autologous personalized neuroregenerative therapy [49].
In view of the limitations of endogenous NSCs and pluripotent stem cells, it is not surprising that the research community has turned its eyes to alternative sources of stem cells with neural regeneration capacity. Of all of them, the ones that seem the best positioned are mesenchymal stem cells (MSCs), which can give rise to all cell lineages of both proper and specialized connective tissues, including bone, cartilage, muscle and adipose cells, among others. MSCs can be extracted from different sources like the bone marrow, the adipose tissue and the umbilical cord [54]. Human dental pulp stem cells (hDPSCs) had also been traditionally included within MSCs, because they fulfill the standard criteria of plastic-adherent growth, multilineage differentiation and a characteristic molecular marker expression as defined by the presence of CD73, CD90 and CD105, which are required by International Society of Cell Therapy to classify a cell type as a MSC [55], in addition to other accessory markers like CD27, CD29, CD44, CD146, CD166, CD271 and STRO-1 [56][57]. This marker expression profile can be found in hDPSCs, as well as in MSCs from many other tissue sources [58]. On the contrary, MSCs and hDPSCs do not express CD45 (hematopoietic marker), CD14 (monocyte or macrophage marker), CD19 (B cell marker) or MHC-II (major histocompatibility complex II) surface molecules [56][57][59]. MSCs have raised substantial hopes for the clinical management of neural lesions, with very promising results [60].
2. DPSCs as Neural Crest Stem Cells. The postnatal DPSC Niche
It soon became apparent that hDPSCs possessed multilineage differentiation potential that exceeded that of conventional MSCs [59][67][68]. Contrary to other MSC sources, the dental pulp tissue is generated by the neural crest, a structure formed at the fusing borders of the neural tube during development. Cells of the neural crest undergo an epithelial-mesenchymal transition and acquire migratory ability, thus extensively colonizing other parts of the embryo, including the pharyngeal arches which are the precursors of craniomaxillofacial organs and tissues (
Figure 1). These neural crest stem cells can subsequently commit to generate the diverse tissues of the oral cavity. Some neural crest stem cells differentiate to MSCs to generate oral neural crest-derived mesenchyme (i.e., ectomesenchyme) which will then give rise to the different oral connective tissues, cartilages, muscles and bones. However, these neural crest stem cells are also the precursors of the cranial peripheral nerve system [69]. Perhaps due to their shared origin, it is not uncommon to observe that hDPSCs express a varied repertoire of both neural progenitor and mature cell markers, even in normal standard (control) culture conditions [59][70][71][72]. Some of the neural markers that are most prominently expressed by hDPSC cultures include Neuroectodermal Stem Cell Intermediate filament marker (Nestin), β-3 tubulin (Tuj1), neurotrophin receptors, and neurofilaments [71][72]. As it can be expected from neural crest-related cells, hDPSC cultures also express neural crest markers like Snail, Slug, Sox10 and HNK1, and also pluripotency-related core factors like Oct4, Sox2 and Nanog [71]. Importantly, the expression of neural crest and pluripotency markers by hDPSCs and the corresponding stemness of these cells can be stimulated by the transient activation of specific signaling pathways, in the absence of any genetic modification [71][73]. Some of these treatments (e.g., Wnt/β-catenin signaling stimulation) have been shown to substantially modify the epigenetic and metabolic footprint of hDPSCs [74][75]. Of particular importance to cell therapy, hDPSCs have a great adaptability to adverse metabolic conditions [76], and can also secrete a large variety of neuroprotective and immunomodulatory factors (discussed in
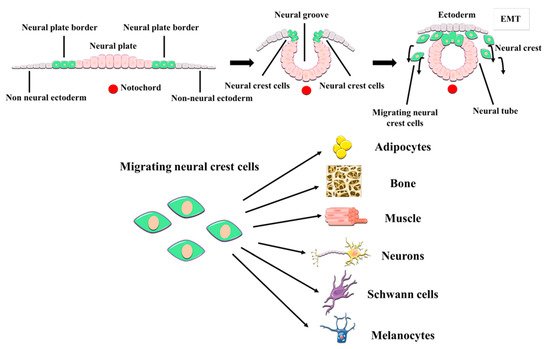
Figure 1. Embryonic origin and multilineage differentiation of human dental pulp stem cells (hDPSCs). hDPSCs derive from neural crest stem cells that generate craniomaxillofacial tissues, including the dental pulp. During development, neural crest cells undergo an epithelial-mesenchymal transition (EMT) and migrate out of the neural tube, to give rise to both mesenchymal and non-mesenchymal cell lineages of the oral cavity, like the neurons and glial cells of the craniofacial PNS. hDPSCs show many neural crest characteristics such as their expression of neural crest markers, and a higher differentiation potential to neural cell lineages than other MSCs.
Figure 2). The niche of DPSCs is thus extraordinarily rich in nerve fibers and blood vessels, in contrast to the surrounding loose connective tissue of the rest of the dental pulp [77]. Immunolabeling of STRO-1 expressing cells revealed that hDPSCs were located precisely within these perivascular niches [78]. Later on, lineage tracing experiments in a murine model revealed that stem cells of the dental pulp were neural crest marker-expressing cells associated with neurovascular bundles [79]. Thus, the same DPSC population could ultimately give rise to both non-mesenchymal (e.g., Schwann cells) and mesenchymal (e.g., odontoblasts) lineage-derived cells [79]. Probably because of their close association with neurovascular structures of the dental pulp, hDPSCs also have a very high capacity to generate vascular cells like endothelia and pericytes [80][81]. This higher ability to differentiate to vascular cells comes at the expense of a reduced capacity for commitment to other more conventional types of mesenchymal-related cell lineages, like chondrocyte differentiation [82]. Thus, according to this model, DPSCs would be located at a similar level to neural crest cells within the stem cell hierarchy, with a higher capacity to generate neural and vascular cells than conventional MSCs. Interestingly, DPSCs also exhibit natural niche homing characteristics when they are engrafted in vivo, as they tend to spontaneously migrate to nerves and vascular structures of the host organism after transplantation [80][83].
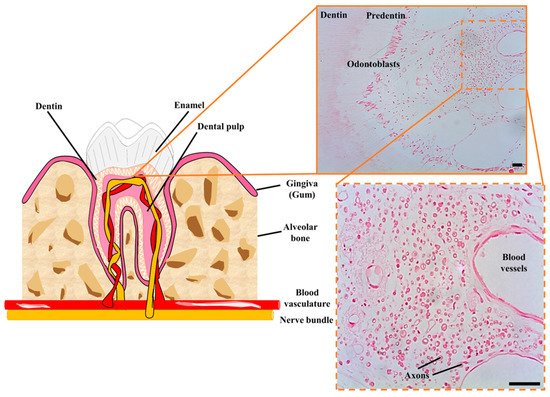
Figure 2. Cellular niche of hDPSCs in postnatal teeth. hDPSCs are harbored in neurovascular bundles of the dental pulp of mature teeth, containing a high concentration of nerve fibers and blood vessels. These neurovascular niches contain many myelinated axons (shown in cross-section) and a higher cellular density than in the rest of the dental pulp tissue. Scale bars: 50 µm.
