You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rahul Shukla | -- | 1251 | 2023-04-17 06:09:29 | | | |
| 2 | Conner Chen | Meta information modification | 1251 | 2023-04-18 08:02:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Epidemiology of Japanese Encephalitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/43099 (accessed on 02 January 2026).
Srivastava KS, Jeswani V, Pal N, Bohra B, Vishwakarma V, Bapat AA, et al. Epidemiology of Japanese Encephalitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/43099. Accessed January 02, 2026.
Srivastava, Kumar Saurabh, Vandana Jeswani, Nabanita Pal, Babita Bohra, Vaishali Vishwakarma, Atharva Ashish Bapat, Yamini Prashanti Patnaik, Navin Khanna, Rahul Shukla. "Epidemiology of Japanese Encephalitis" Encyclopedia, https://encyclopedia.pub/entry/43099 (accessed January 02, 2026).
Srivastava, K.S., Jeswani, V., Pal, N., Bohra, B., Vishwakarma, V., Bapat, A.A., Patnaik, Y.P., Khanna, N., & Shukla, R. (2023, April 17). Epidemiology of Japanese Encephalitis. In Encyclopedia. https://encyclopedia.pub/entry/43099
Srivastava, Kumar Saurabh, et al. "Epidemiology of Japanese Encephalitis." Encyclopedia. Web. 17 April, 2023.
Copy Citation
Japanese encephalitis virus (JEV) is the causal agent behind Japanese encephalitis (JE), a potentially severe brain infection that spreads through mosquito bites. JE is predominant over the Asia-Pacific Region and has the potential to spread globally with a higher rate of morbidity and mortality. Efforts have been made to identify and select various target molecules essential in JEV’s progression.
Japanese encephalitis (JE)
antiviral
vaccine
drug
1. Introduction
Japanese encephalitis virus (JEV) is the predominant cause of viral encephalitis in Asia [1]. It is a mosquito-borne flavivirus that is the causative agent of Japanese encephalitis [2]. Japanese encephalitis (JE) was reported for the first time in 1871, in Japan, and JEV was first isolated in 1935 from the brain of a fatal case of JE. This isolate, known as the Nakayama strain, is acknowledged as the prototype strain of JEV [3]. Other clinically relevant viruses belonging to the same genus include dengue virus (DENV), yellow fever virus (YFV) Murray Valley encephalitis (MVE), West Nile virus (WNV), zika virus (ZIKV), St. Louis encephalitis virus (SLEV) and tick-borne encephalitis virus (TBEV) [4]. According to a WHO report published in 2019, almost 68,000 cases of JE with a 20–30% mortality rate were recorded annually. A study based on mathematical modeling with age-stratified case data estimated that approximately 100,308 clinical cases and 20,000–30,000 deaths occurred due to JEV in 2015 across the globe [5]. Newborns and children up to the age of 15 years are more vulnerable to JE with the increased threat of neurological complications over adults [6]. Almost 2 billion people living in endemic countries face a constant threat of JE and an upsurge in the mosquito population poses a risk of expansion to newer geographical areas.
The JEV is an RNA virus, primarily transmitted through the bite of an infected female mosquito, Culex tritaeniorhynchus. Other Culex species, such as Cx. annulirostris, Cx. vishnui, Cx. pseudovishnui, Cx. gelidus, Cx. sitiens and Cx. fuscocephela are also reported to be involved in the transmission of JEV along with some Anopheles mosquito species, such as Anopheles subpictus, An. peditaeniatus and An. hyrcanus [7]. The primary reservoirs of JEV are the birds of the family Ardeidae, such as herons and egrets. Pigs are highly susceptible to JEV, where the virus becomes amplified in optimum levels and they develop a high circulating viral titer (amplified host), and are therefore able to spread the infection to naive mosquitoes [8]. Reports suggest that at this stage, pigs tend to shed the virus in oronasal secretion and may potentiate the horizontal transmission of JEV [9]. Unlike pigs and birds; humans, cattle and horses do not develop high viral titers, making them ‘dead-end’ hosts, as shown in Figure 1.
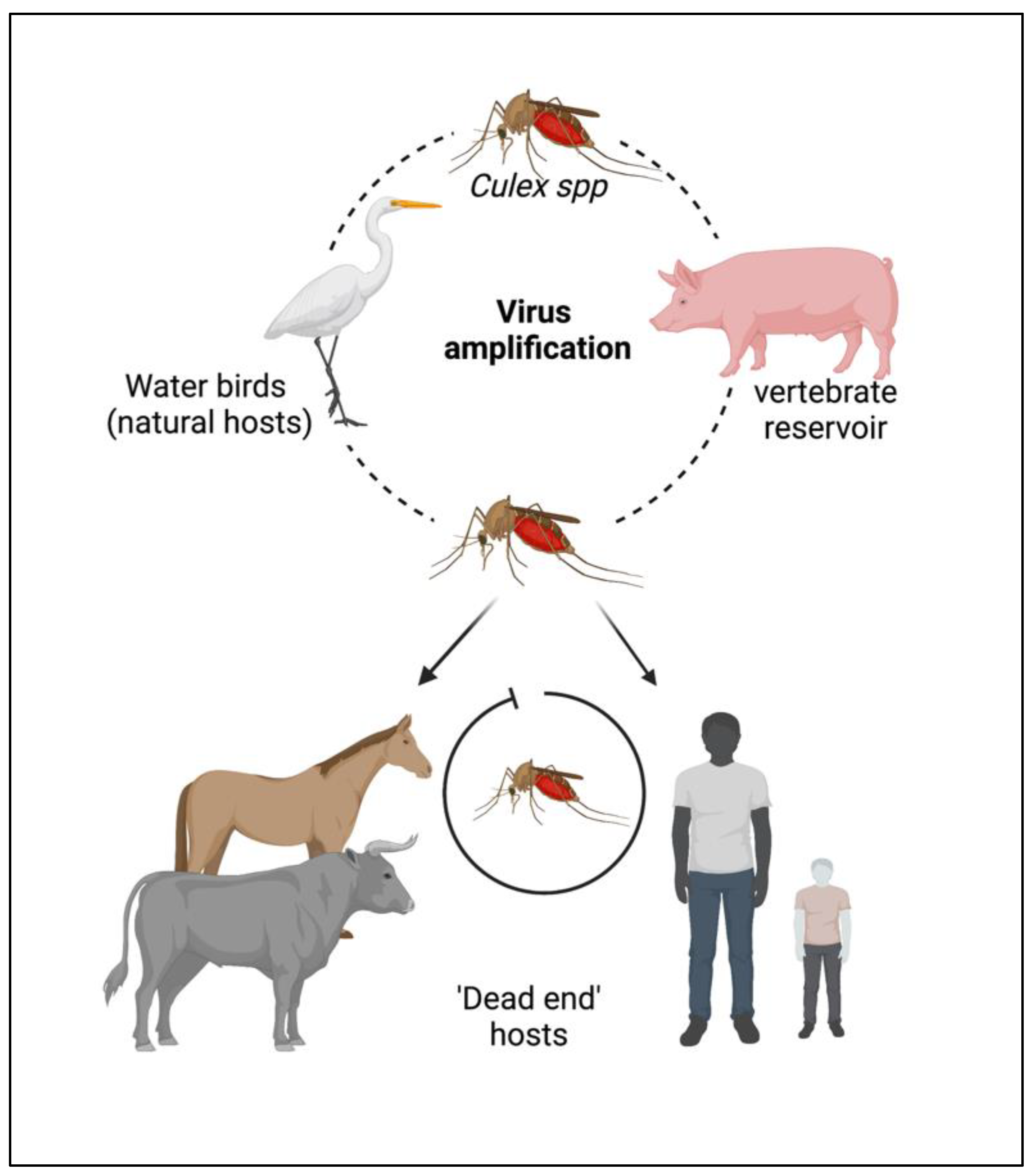
Figure 1. Cycle of the Japanese encephalitis virus (JEV) infection and amplification. Long-legged water birds, such as herons, storks, and ibises are the primary reservoirs and natural hosts for JEV. JEV-infected female mosquitoes, especially Culex tritaeniorhynchus, transmit the virus from wading water birds to other animals (pigs, cattle, and other hooved animals) and humans. Pigs act as secondary hosts where the virus becomes amplified at an optimum level and carries the infectious virion from one place to another (vector-free transmission). Female Culex mosquitos take up the virus from here and infect humans by biting them. The infected humans act as ‘dead-end’ hosts for the virus as JEV does not develop a titer high enough in the blood circulation to transmit through feeding mosquitoes.
Nonetheless, JEV has an enzootic cycle, due to which the virus can persist in nature to such an extent that it might be next to impossible to eradicate it in the near future. Thus, effective antiviral therapy and an ideal vaccine against JEV are of pressing priority. Despite challenges, such as the lack of an appropriate drug delivery system or a treatment plan independent of the stage of infection, JE research has increased rapidly with growing technological advancements, with the primary aim of developing a safe and cost-effective therapeutic (drugs and vaccines) for all age groups.
2. Epidemiology
The majority of cases of viral encephalitis in the Asian subcontinent are due to JEV [6]. This spans a large region that includes majorly tropical parts of Asia, such as Japan, China, Taiwan, Korea, the Philippines, India and all of Southeastern Asia. Countries with confirmed JE epidemics include India, Nepal, Pakistan, Sri Lanka, Myanmar, Laos, Vietnam, Malaysia, the Philippines, Singapore, China, Indonesia, maritime Siberia, Japan and Korea [10]. Additionally, sporadic outbreaks in the Western Pacific and northern Australia are also observed [6]. Historically, outbreaks similar to JE had been recorded in Japan in the late 1800s; however, the first confirmed JE case was documented in 1924 in Japan, followed by Korea (1933), China (1940), the Philippines (1950), India (1955) and many other Asian countries thereafter [5]. In recent decades, geographical hotspots for JE incidences have shifted considerably from South Asian countries (e.g., Japan, South Korea and Taiwan) to South East Asian countries, including Bangladesh, Cambodia, India, Indonesia and Pakistan (Figure 2).
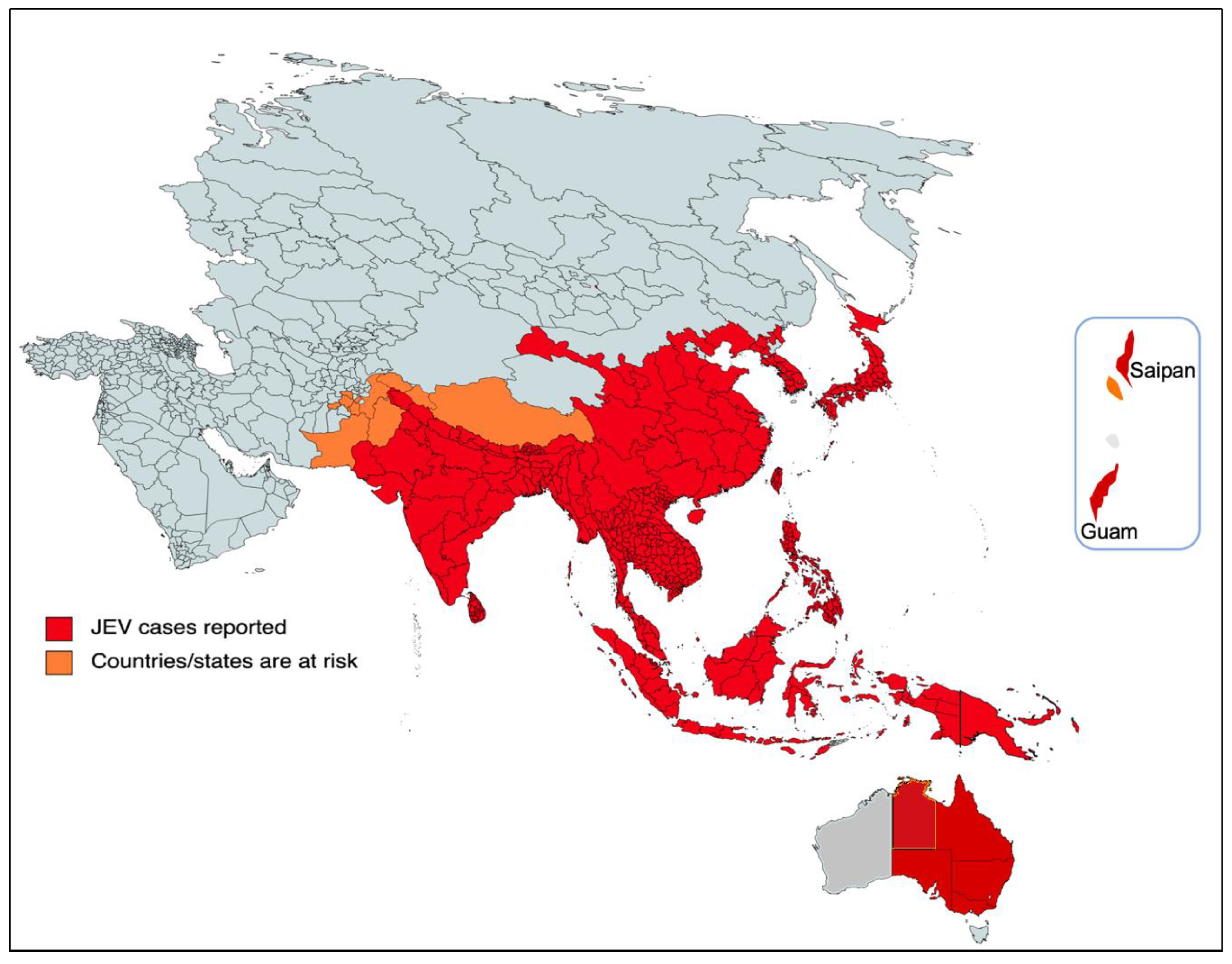
Figure 2. Geographical distribution of JEV. The red part of the focused Indo-Pacific geographical regions and countries indicates where active JEV cases have been reported since its outbreak. The orange color shows the areas that have the highest risk of JEV infection in the near future. The epidemiological data of JEV was modified from https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 26 December 2022).
Since the early 1970s, there has been a rise in epidemic activity of JEV in the Indian subcontinent. Then, in late 1990s, the virus persisted to spread into the neighboring territories of southern Pakistan along with the Kathmandu Valley of Nepal [10].
In India, the first evidence of JE was obtained through serological studies back in 1952, and the first JEV case in India was documented in 1955. Later on, frequent outbreaks were reported in all parts of India at regular intervals [10][11]. A major outbreak occurred in the Bankura district of West Bengal in 1973, with a fatality rate of 42.6% [10]. The most prolonged epidemic of JE was witnessed in the Gorakhpur district of Uttar Pradesh in 2005, with more than 5500 documented cases of viral encephalitis and ~23% fatalities [10][12][13]. Several reports indicated the expansion of the virus to the newer non-endemic areas, including the northern and northeastern parts of the Indian continent, and cases have been reported that signify the spread of the virus, including in urban areas, such as New Delhi [14][15].
More recently, JEV infection has made a threat of resurgence that was highlighted by the fairly large epizootic outbreak in Australia in 2022 [16]. In Australia, the first JE case was identified in 1995 and the virus has remained dormant over the past two decades [17]. In early 2021, it reappeared and was diagnosed in the resident of the northern territory in Queensland, which resulted in death, and there appeared to be a sentinel event in the recent outbreak in 2022 in the southern Australian states. Then, JEV was detected in stillbirths, mummified fetuses and newborn piglets from several commercial piggeries majorly located in the four southern states of Australia (New South Wales, Queensland, South Australia, and Victoria), Figure 2. As per the Australian government, 45 human cases of JEV have been notified up to 5 January 2023, of which 35 are confirmed cases with conclusive clinical evidence and seven fatalities [16][18][19]. The primary causative agents of the JE transmission were the members of the subgroup Culex sitiens, particularly Cx. annulirostris [9]. The other Culex species, Cx. quinquefasciatus, Cx. gelidus and Cx. tritaeniorhynchus were suspected to transmit the disease in Australia [18], although more research and mosquito surveillance needs to be carried out to confirm their geographic distribution and abundance in Australia. Nonetheless, current JEV infections in the southern Australian states pose a significant risk to their neighboring territorial states, such as the Northern Territory and Western Australia.
References
- Burke, D.S.; Leake, C.J. Japanese encephalitis. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 3, pp. 63–92.
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3.
- Mackenzie, J.S.; Williams, D.T.; Hurk, A.F.V.D.; Smith, D.W.; Currie, B.J. Japanese Encephalitis Virus: The Emergence of Genotype IV in Australia and Its Potential Endemicity. Viruses 2022, 14, 2480.
- Billoir, F.; de Chesse, R.; Tolou, H.; de Micco, P.; Gould, E.A.; de Lamballerie, X. Phylogeny of the genus Flavivirus using complete coding sequences of arthropod-borne viruses and viruses with no known vector. J. Gen. Virol. 2000, 81, 781–790.
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. Elife 2020, 9, e51027.
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774.
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel Med. 2018, 25, S16–S26.
- Ricklin, M.E.; García-Nicolás, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Nougairede, A.; Charrel, R.N.; Posthaus, H.; Oevermann, A.; Summerfield, A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016, 7, 10832.
- Chiou, S.-S.; Chen, J.-M.; Chen, Y.-Y.; Chia, M.-Y.; Fan, Y.-C. The feasibility of field collected pig oronasal secretions as specimens for the virologic surveillance of Japanese encephalitis virus. PLoS Negl. Trop. Dis. 2021, 15, e0009977.
- Tiwari, S.; Singh, R.K.; Tiwari, R.; Dhole, T.N. Japanese encephalitis: A review of the Indian perspective. Braz. J. Infect. Dis. 2012, 16, 564–573.
- Kulkarni, R.; Sapkal, G.N.; Kaushal, H.; Mourya, D.T. Japanese Encephalitis: A Brief Review on Indian Perspectives. Open Virol. J. 2018, 12, 121–130.
- Prakash, P.J.; Kusum, V.; Vijeta, S. Japanese Encephalitis (JE): A curse for people living in Uttar Pradesh, India. J. Vaccines Immunol. 2021, 7, 036–040.
- Solomon, T. Control of Japanese encephalitis—Within our grasp? N. Engl. J. Med. 2006, 355, 869–871.
- Walsh, M.G.; Pattanaik, A.; Vyas, N.; Saxena, D.; Webb, C.; Sawleshwarkar, S.; Mukhopadhyay, C. High-risk landscapes of Japanese encephalitis virus outbreaks in India converge on wetlands, rain-fed agriculture, wild Ardeidae, and domestic pigs and chickens. Int. J. Epidemiol. 2022, 51, 1408–1418.
- Singh, L.S.; Singh, H.L.; Thokchom, N.; Singh, R.M. A Descriptive Study on Prevalence Pattern of Japanese Encephalitis in State of Manipur. Indian J. Med. Microbiol. 2019, 37, 235–240.
- Australian Government Department of Health and Aged Care. Japanese Encephalitis Virus (JEV). Available online: https://www.health.gov.au/health-alerts/japanese-encephalitis-virus-jev/about#current-status (accessed on 25 January 2023).
- Hurk, A.F.V.D.; Pyke, A.T.; Mackenzie, J.S.; Hall-Mendelin, S.; Ritchie, S.A. Japanese Encephalitis Virus in Australia: From Known Known to Known Unknown. Trop. Med. Infect. Dis. 2019, 4, 38.
- Hurk, A.F.V.D.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208.
- Waller, C.; Tiemensma, M.; Currie, B.J.; Williams, D.T.; Baird, R.W.; Krause, V.L. Japanese Encephalitis in Australia—A Sentinel Case. N. Engl. J. Med. 2022, 387, 661–662.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
18 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




